HybridSPE®-Phospholipid Technology
Effective Removal of Phospholipids and Proteins for Accurate and Reproducible LC-MS Analysis
Section Overview
- HybridSPE®-PLus Plates - the Next Generation in HybridSPE®-Phospholipid Technology
- Impact of Phospholipids on Accuracy and Reproducibility of LC-MS Data
- Limitations of Traditional Sample Prep Methodologies for Biological Samples
- The Unique Chemistry and Basic Methodology of HybridSPE®-Phospholipids Technology
HybridSPE®-PLus Plates - the Next Generation in HybridSPE®-Phospholipid Technology
Our new HybridSPE®-PLus plates offer the same time-proven chemistry of our traditional HybridSPE®-Phospholipid plates coupled with plate manufacturing enhancements that stem from years of production experience. These manufacturing enhancements have allowed us to increase well-to-well flow consistency and reduce sample hold-up volumes, further improving analyte recoveries and assay reproducibility. We will continue to offer and support our traditional square-well HybridSPE®-Phospholipid plates for those customers who have developed methods on them, but we invite those customers developing new methods to take advantage of the newest technology: HybridSPE®-PLus.
Impact of Phospholipids on Accuracy and Reproducibility of LC-MS Data
Phospholipids: A Concern for LC-MS Analysis of Small Molecules in Biological Matrices
Phospholipids are present as a major component of all cell membranes. They are therefore present in all biological sample matrices including serum, plasma and whole blood and can be a problem in LC-MS analysis of small molecules because they often co-elute and ionize along with the analytes of interest. This co-elution results in ion suppression (an erroneous decrease) of the mass spec signal that can cause variability and impact LC-MS result accuracy (Figure 1).

Figure 1. Phospholipid Effect on Ionization of Clonidine
Importance of Accurate Results and Fast Answers in Bioanalysis
We understand that the results of your analyses can have a significant impact on the lives of many others. This puts pressure on you to ensure the data you produce is as accurate as possible. Not only do you need quick answers, you need answers you can trust. The complexity of the types of samples with which you work does not make your job any easier. Proteins and phospholipids inherently present, to varying degrees in your samples, can add variability to your analytical results when using sensitive techniques such as LC-MS or LC-MS/MS. We have over four decades of chromatography expertise to help you navigate the complexity of your sample prep options.
Limitations of Traditional Sample Prep Methodologies for Biological Samples
Limitations of Traditional Biological Sample Cleanup Methods
Most clinical and biological researchers use traditional methods such as protein precipitation and liquid-liquid extraction to cleanup their samples prior to analysis. While these techniques allow for inexpensive and quick removal of proteins, they completely fail to address the problem of phospholipid-induced ion suppression.
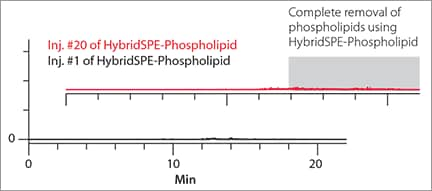
Even if phospholipids do not co-elute with the analyte of interest, they can accumulate on your analytical column and elute from the column sporadically in downstream analyses. This can cause unpredictable ion suppression and poor reproducibility, thereby putting the accuracy of your results at risk (Figure 2).
Background Interference Observed after Multiple Injections Using Std. Protein PPT

Figure 2. Gradient RP LC-MS of Blank Plasma Samples Prepared by Standard Protein PPT vs. HybridSPE®-Phospholipid
Background Interference Observed after Multiple Injections Using HybridSPE®-Phospolipid Technology
Back Pressure Generated Within C18 1.8 µm Column (5 cm x 2.1 mm) after Multiple Injections

Phospholipid Removal Techniques
To overcome the problem of phospholipid-induced ion suppression, some analysts try traditional SPE. Traditional SPE often requires time-consuming and complex method development, but still only removes nominal amounts of phospholipids. Remaining phospholipids can impact the accuracy of your results, accumulate on your analytical column to impact future analyses, add to column replacement costs and increase instrument downtime.
A variety of products designed specifically for the removal of both proteins and phospholipids are now commercially available, including HybridSPE® plates and cartridges. Most of these products are simple, fast and easy-to-use, offering fairly standardized methods with minimal method development. Most, however, use a hydrophobic retention mechanism to separate phospholipids from analytes of interest in the sample. This poses a problem if the analytes of interest are also hydrophobic because they will be retained and removed along with the hydrophobic phospholipids. This results in decreased analyte recovery and inaccurate results. HybridSPE®Phospholipid Technology is different in that it completely removes both proteins and phospholipids from the sample without retaining other hydrophobic compounds.
The Unique Chemistry and Basic Methodology of HybridSPE®-Phospolipid Technology
How Is HybridSPE®-Phospholipid Technology Different Than Other Phospholipid Removal Products?
The first of its kind, HybridSPE®-Phospholipid technology was introduced in 2008. It fuses the simple, standardized methodology of traditional protein precipitation with the specificity of solid phase extraction (SPE) for the simultaneous removal of proteins and phospholipids from biological samples prior to LC-MS analysis. Unlike other phospholipid removal products that use a hydrophobic retention mechanism to remove phospholipids from biological samples, HybridSPE®-Phospholipid technology uses a unique retention mechanism (Figure 3). This allows it to separate phospholipids from even very hydrophobic analytes, such as vitamins which are often retained along with phospholipids on competitive products.

Figure 3. Unique Retention Mechanism of HybridSPE®-Phospholipid Technology Allows for Separation of Even Very Hydrophobic Analytes from Phospholipid Contaminants in Biological Sample Matrices
A Newer, Better “Go-to” Sample Prep Method for Phospholipid Removal
Labs working with biological samples often choose to perform protein precipitation prior to LC-MS analysis, using it as their “go-to” sample prep method. They view phospholipid removal as more costly, more time-consuming and unnecessary if the specific analytes of interest do not co-elute with the phospholipids in their sample. This is not the case.
Labs can actually reduce overall costs and increase overall throughput while generating more accurate data by using HybridSPE®-Phospholipid technology to remove both proteins and phospholipids from all biological samples prior to small molecule LC-MS analysis.
Simple and Fast Phospholipid Removal
The 96-well plate protocol involves just a few simple steps (Figures 4 and 5), and plates can be used right out of the package with no pre-conditioning step required. The sample (containing internal standards if desired) and a precipitation solvent are first added to the well plate, followed by a mixing step and vacuum application to collect the sample. Collected samples are then ready to be analyzed.

Figure 4. Depiction of Basic HybridSPE®-Phospholipid Sample Prep Workflow
1. Add Sample Pipette 100 µL plasma or serum to the HybridSPE® plate followed by 300 µL precipitation solvent. Add internal standards as necessary. |
2. Mix by vortexing/shaking HybridSPE® plate or by aspirating/dispensing with 0.5–1 mL pipette tip. |
3. Apply vacuum The packed-bed filter/frit assembly acts as adepth filter for the concurrent physical removal of precipitated proteins and chemical removal of phospholipids. Small molecules (e.g., pharma compounds and metabolites) pass through unretained. |
4. Collect Sample Resulting filtrate/eluate is free of proteins and phospholipids and ready for immediate LC-MS/MS analysis. |

Figure 5. HybridSPE® 96-well Plate Protocol
Related Resources
- Data Sheet: Instructions for HybridSPE®-PLus 96-Well Plates
Phospholipids cause ion suppression in LC-MS analyses and can accumulate on columns, affecting accuracy and increasing costs. HybridSPE-Phospholipid technology effectively removes these interferences, improving the reliability of LC-MS results.
- Data Sheet: Instructions & Troubleshooting for HybridSPE®-Phospholipid Ultra Cartridge
The HybridSPE®-Phospholipid Ultra Cartridge cleans biological samples for LC-MS-MS by removing small particles, proteins, and phospholipids that cause ion suppression, utilizing a selective interaction between zirconia ions and phospholipids.
- Webinar: Meeting the LC-MS Needs of a High-Throughput Clinical Lab
The rise of LC-MS/MS in clinical laboratories has created challenges for service providers and vendors due to differing workflows and analytes. This presentation will discuss alternative sample extraction and chromatography solutions for endocrinology and biochemical genetics in hospital settings.
- Product Information: HybridSPE®-Precipitation Technology
In pharmaceutical bioanalysis, phospholipid contamination causes ion suppression during drug quantification in biological fluids, impacting signal detection and leading to uncontrolled elution in LC runs. HybridSPE-Precipitation provides a simple solution to remove these interferences.
- Article: Enrichment of Phospholipids in Biological Samples Using HybridSPE®
A simple method to enrich phospholipids from plasma samples, involving a HybridSPE-PPT 96-well plate that both retains phospholipids and removes precipitated proteins.
- PDF: High Recovery Method of HybridSPE®-Phospholipid for Cleanup of Biological Samples Prior to LC-MS Analysis
The method includes in-well protein precipitation using organic solvents like acetonitrile and methanol, along with phospholipid removal using proprietary zirconia-modified silica particles. This process is both simple and fast.
- PDF: High Throughput Bioanalysis Utilizing Fused-Core™ Particle and HybridSPE®-PPT Technology (HPLC 2010)
The trend in high-throughput bioanalysis favors faster chromatographic separations with minimal sample preparation, which can compromise data quality. This presentation highlights recent advancements in chromatographic media and sample preparation techniques that enhance throughput while improving data quality and simplifying methods.
- PDF: Alternative Method of Hybrid SPE Sample Preparation for High Recovery LC-MS Analysis of Pharmaceutical Compounds in Plasma
This sample cleanup method efficiently removes proteins and phospholipids using organic solvents for protein precipitation and proprietary zirconia particles for phospholipid removal. It is simple, fast, and suitable for high-throughput applications.
- PDF: Comparison of Sample Preparation Techniques for Reduction of Matrix Interference in Biological Analysis by LC-MS-MS
Matrix effects in biological samples can cause variability in LC-MS due to co-elution of phospholipids with analytes. This study evaluates three sample preparation techniques for effective phospholipid removal, optimizing the HybridSPE method while using generic SPE and PPT methods without modification.
- PDF: Depletion of Phospholipid Matrix Interference when Dealing with Small Volume Plasma Samples
Sample preparation of small plasma volumes (e.g., 30 µL from mice) is challenging due to large hold-up volumes in solid phase extraction and increased matrix interference from liquid-liquid protein precipitation. This affects analyte quantitation and requires time-consuming gradient elution to mitigate matrix effects.
- PDF: High Throughput Removal of Both Phospholipids and Proteins in Bioanalytical Sample Preparation
Sample preparation is vital in pharmaceutical bioanalysis due to complex matrices. Techniques like protein precipitation and solid phase extraction (SPE) are used, with SPE being selective but time-consuming. Protein precipitation removes only proteins, leaving phospholipids that can cause ion suppression and affect results.
- PDF: Impact of Ion-Suppression Due to the Presence of Phospholipids on the Enantiomeric LC-MS Analysis of Clenbuterol
This study shows the impact of ion suppression on enantiomeric separation with standard protein precipitation (PPT) and correlates it with analyte concentration. It also highlights the HybridSPE-PPT protocol for phospholipid removal using clenbuterol's racemic mixture.
- PDF: Increased Bioanalytical Throughput Utilizing Fused-Core™ Particles with Selective Phospholipid Depletion
The HybridSPE™ platform improves chromatographic speed and throughput by selectively depleting phospholipids. This study evaluates the HybridSPE Small Volume 96-well plate for 20-40 µL rat plasma samples, featuring a zirconia-coated silica phase and a 0.45 µm polishing filter.
- PDF: Recovery of Pharmaceutical Drugs From Small Volume Biological Sample Using HybridSPE®-PPT 96-well Plate
The HybridSPE-PPT method integrates protein precipitation and phospholipid removal to enhance sample preparation efficiency. It emphasizes optimizing elution volume and reproducibility, applicable in various bioanalytical contexts.
- PDF: Selective Depletion of Phospholipids Interference Utilizing HybridSPE™-Precipitation Technology
Biological sample analysis is hindered by interferences from preparation techniques. Protein precipitation can cause chromatographic irregularities due to co-extracted phospholipids, while solid phase extraction (SPE) offers better cleanup but increases time and complexity.
계속 읽으시려면 로그인하거나 계정을 생성하세요.
계정이 없으십니까?