Low Bleed
The Proof: Low Bleed
The low bleed character of SLBms columns is the result of Unique Advances in Polymer Synthesis and the Innovative Manufacturing Processes that we established for these columns.
Column bleed is a result of stationary phase fragments releasing from the inside wall of the column. This adversely affects chromatographic performance by raising detection limits, reducing the quality of mass spectra, and increasing the need for preventative maintenance. For these reasons, columns with low bleed characteristics are desirable.
Low Bleed = High Signal-to-Noise Ratio = Low Detection Limits
Low levels of detection are a requirement of chromatographers in many different fields. Environmental chemists must meet stringent reporting requirements. Analysts in the pharmaceutical, food & beverage, and personal product industries must ensure that harmful compounds are not present in consumer goods. Material scientists must fully characterize raw materials. Analysts rely on GC-MS and other GC methods to enable highly sensitive, low level detection. When measurement is required at the ppb or even ppt level, extreme care must be taken to ensure that nothing interferes with the analysis.
System noise hinders sensitivity by lowering the signal-to-noise ratio. System noise can originate from many different sources in a GC system. Historically, GC column bleed has been a significant source of system noise. For this reason, today's chemists require capillary columns that exhibit a very low level of bleed in addition to being inert towards the various analytes in the method.
US EPA Method 8270 for semivolatiles by GC-MS is a formidable challenge for column bleed because of the low detection limits required by the method and also the varying functionality of the analytes. The ideal column should be inert enough to provide excellent peak shape and also exhibit very low bleed. Figure 1 shows a US EPA Method 8270 analysis on an SLB-5ms column and -5ms columns from three competitors that are advertised as low bleed and MS-suitable. Figure 2 is a bar chart representation of this same data. As is evident, the SLB-5ms column has lower column bleed than any of the competitor columns.
All the columns used to generate the data presented below in Figures 1-8 were conditioned for 45 minutes after installation in the instrument. Eight replications of a 5 ng on-column standard were performed on each column. Column bleed measurements were taken at the 325 °C isothermal portion of the eighth run. Mass spectra of benzo(g,h,i)perylene, which elutes during the 325 °C isothermal portion of the run, were also taken from the eighth run.
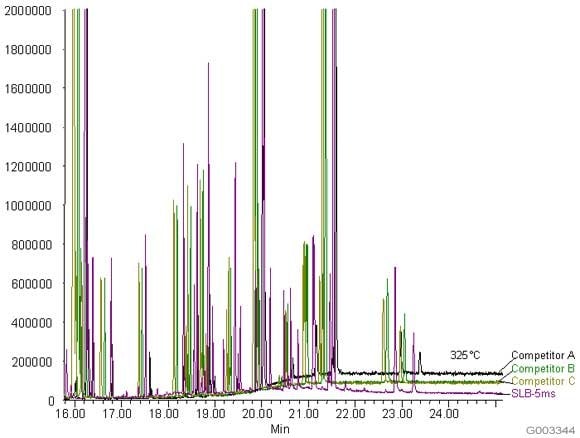
Figure 1.Comparison of Baselines from an SLB-5ms and Three Competitive -5ms Columns
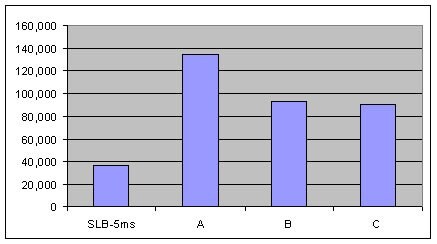
Figure 2.Comparison of MSD Bleed Measured as Total Ion Current (TIC) at 325 °C from an SLB-5ms and Three Competitive -5ms Columns (Conditions same as Figure 1).
The bar chart in Figure 3 shows the signal-to-noise ratio of the TIC peak for benzo(g,h,i)perylene at 325 °C, when the level of column bleed is the highest. Data is presented for the same columns used in Figure 1. High noise levels are undesirable since they make it difficult for the integration software to adequately measure all of the peak area. The better the signal-to-noise ratio, the more area counts are obtained, which results in the ability to achieve lower detection limits.
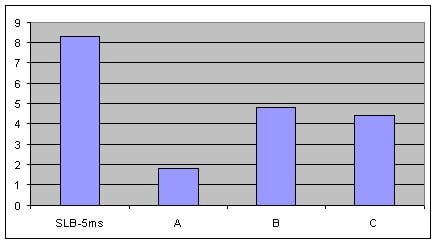
Figure 3.Comparison of Signal-to-Noise Ratio for Benzo(g,h,i)perylene from the TIC at 325 °C on an SLB-5ms and Three Competitive -5ms Columns, 8th Injection (Conditions same as Figure 1)
Low Bleed = Clean Mass Spectra = Easy Mass Spectral Identification
Analysts using MS detection have an additional concern: high column bleed interfering with proper mass spectral identification of analytes. MS programs measure the abundances of ions formed from the fragmentation of analytes. The most abundant ion is depicted as 100% height, while all other ions are represented as a percentage of its height. The most abundant ion is typically used as the “quant ion” for quantitative purposes. The other, qualifier, ions are used for qualitative purposes. For the software to assign a high probability of positive identification, the qualifier ions must be within specific ratio ranges relative to the quant ion when comparing a sample mass spectrum to a mass spectral library. If extraneous ions, such as from column bleed, are present in the sample mass spectrum, the software will assign a lower probability of positive identification.
Additionally, many US EPA Methodologies require GC-MS analysts to assign tentative identification to non-target “unknown” compounds which may also be present in the sample extract. These are called Tentatively Identified Compounds, or TICs. In order for the software to assign a high probability of positive identification, the mass spectrum from the sample extract must compare well with the mass spectral library entry. High column bleed levels interfere with this comparison, resulting in the reporting of TICs that are either poorly identified or misidentified.
As a measure of column bleed, the mass spectrum of the last eluting peak, benzo(g,h,i)perylene, was examined at a 5 ng on-column level, on an SLB-5ms column and -5ms columns from three competitors that are advertised as low bleed and MS-suitable. This is a good indicator of a column’s bleed characteristic since this analyte elutes following the thermal gradient profile, when the level of column bleed is the highest. The major column bleed ion, m/z = 207, resulting from the formation of cyclic hexamethylcyclotrisiloxane (D3), should be present at a low level relative to the quant ion, m/z = 276. Additionally, this column bleed ion should be at a level lower than m/z = 138 and m/z = 277, two qualifier ions used to confirm the identity of the peak.
Figure 4 is a bar chart depicting ratio data (major column bleed ion vs. quant ion) while Figures 5-8 show the mass spectra obtained from the Figure 1 chromatograms. The SLB-5ms column showed negligible column bleed compared to the competitive columns. No competitive column had bleed levels as low as the SLB-5ms. The column bleed from the competitor “A” column was the highest, with the column bleed ion actually larger than the two qualifier ions. What does all this mean? The MS software would assign a low probability of positive identification for benzo(g,h,i)perylene for analyses using the competitor “A” and “C” columns. For TICs, the situation would be even less desirable since retention time data would not be available to assist with identification.
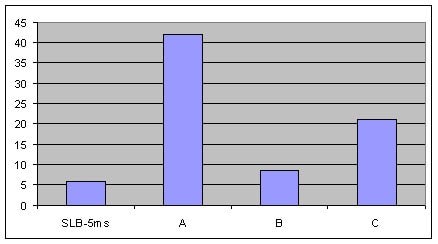
Figure 4.Comparison of MSD Bleed Measured as the % of the Column Bleed Ion, m/z = 207, to the Quant Ion, m/z = 276, in the Mass Spectra of Benzo(g,h,i)perylene at 325 °C from an SLB-5ms and Three Competitive -5ms Columns, 8th Injection (Conditions same as Figure 1)
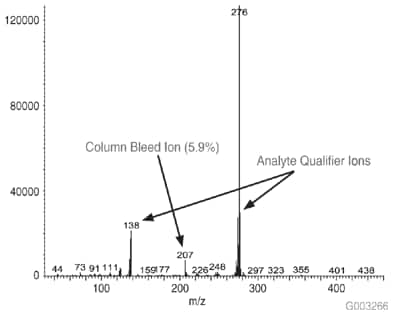
Figure 5.Mass Spectrum of 5 ng On-column of Benzo(g,h,i)perylene at 325 °C on an SLB-5ms Column, 30 m x 0.25 mm I.D., 0.25 µm, 8th Injection (Conditions same as Figure 1)
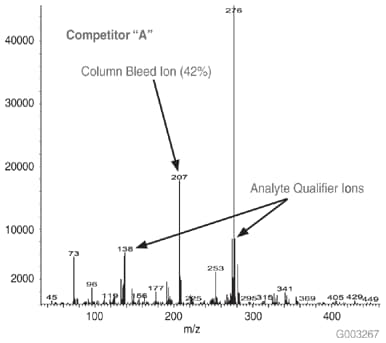
Figure 6.Mass Spectrum of 5 ng On-column of Benzo(g,h,i)perylene at 325 °C on a -5ms Column from Competitor A, 30 m x 0.25 mm I.D., 0.25 µm, 8th Injection (Conditions same as Figure 1)
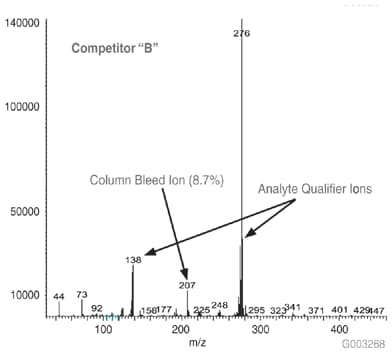
Figure 7.Mass Spectrum of 5 ng On-column of Benzo(g,h,i)perylene at 325 °C on a -5ms Column from Competitor B, 30 m x 0.25 mm I.D., 0.25 µm, 8th Injection (Conditions same as Figure 1)
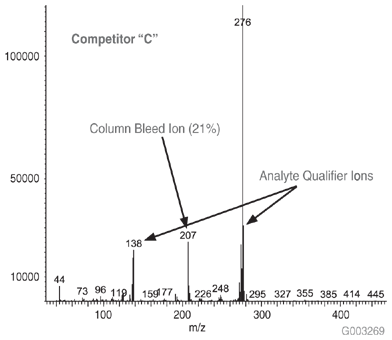
Figure 8.Mass Spectrum of 5 ng On-column of Benzo(g,h,i)perylene at 325 °C on a -5ms Column from Competitor C, 30 m x 0.25 mm I.D., 0.25 µm, 8th Injection (Conditions same as Figure 1)
Low Bleed = Negligible Detector Contamination = Minimal Instrument Downtime
High column bleed can foul the GC detector, reducing detector sensitivity. When the fouling becomes severe enough to warrant action, the detector must be dismantled and cleaned, a procedure that may remove an instrument from service for one or more days. MSD lenses can become coated and require polishing. The active foil in an ECD can become fouled to the point the entire detector needs to be sent out for refurbishing. Partially plugged FID jets need to be replaced. PID windows can become layered with contamination and require cleaning.
Instrument downtime means samples cannot be processed, resulting in a backlog, something that most analysts cannot afford.
계속 읽으시려면 로그인하거나 계정을 생성하세요.
계정이 없으십니까?