Fingolimod HPLC Assay & Impurity Profiling (USP)
Introduction
A rapid, accurate, and simple method was implemented for the total chromatographic purity analysis of Fingolimod Hydrochloride by High Performance Liquid Chromatography equipped with a Diode Array Detector. The experimental conditions follow guidelines, with minor allowed modifications, from the USP43-NF38 monograph methods for Fingolimod Hydrochloride Assay and Organic Impurities Profiling. Fingolimod hexyl homolog, Fingolimod heptyl homolog, Fingolimod Hydrochloride, Fingolimod nonyl homolog, Fingolimod decyl homolog, 3-Phenethyl Fingolimod analog, and 2-Phenethyl Fingolimod analog can be resolved with baseline separation utilizing the USP‘s 33-minute gradient method and a Purospher STAR RP-18 HPLC column (150 x 3.0 mm, 3 μm). A 0.1% solution of Phosphoric acid in water and acetonitrile were employed as the mobile phases for the gradient elution. Under applied conditions, system suitability criteria are met, and the method demonstrates good resolution/selectivity, reproducibility, and sensitivity.

Figure 1.Fingolimod HCl

Figure 2.Fingolimod Hexyl Homolog

Figure 3.FingolimodHeptyl Homolog

Figure 4.O-Acetyl Fingolimod

Figure 5.Fingolimod Nonyl Homolog

Figure 6.Fingolimod Decyl Homolog

Figure 7.Fingolimod 3-Phenethyl Analog
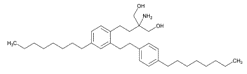
Figure 8.Fingolimod 2-Phenethyl Analog
|
|---|




계속 읽으시려면 로그인하거나 계정을 생성하세요.
계정이 없으십니까?