모든 사진(1)
About This Item
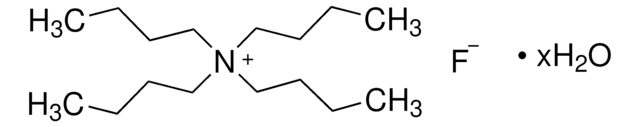
Linear Formula:
[CH3(CH2)3]4NF · 3H2O
CAS Number:
Molecular Weight:
315.51
Beilstein:
3761900
EC Number:
MDL number:
UNSPSC 코드:
12352107
PubChem Substance ID:
NACRES:
NA.22
추천 제품
일반 설명
Tetrabutylammonium fluoride trihydrate is a mild base used in reactions like aldol-type condensation reactions, Michael-type reactions, ring-opening reactions. Its is also used as a promoter in cross-coupling reactions and cyclization of carbocycles and heterocycles.
애플리케이션
Reactant for:
Preparation of deprotecting agents in preparation of cellulose derivatives
Synthesis of lipophilic peptides for DNA transfections in vivo
Dehydrobromination reactions
Preparation of deprotecting agents in preparation of cellulose derivatives
Synthesis of lipophilic peptides for DNA transfections in vivo
Dehydrobromination reactions
Tetrabutylammonium fluoride trihydrate can be used as a base:
It can be used to catalyze ethynylation of quinolines and isoquinolines using calcium carbide in aqueous N,N-dimethylacetamide.
- For the dehydrobromination of vinyl bromides to terminal acetylenes.
- In the conversion of 1,1-dibromo-1-alkenes to terminal alkynes via Corey–Fuchs reaction.
- In Hiyama cross-coupling reaction of aryl and heteroaryl chlorides with aryltrialkoxysilanes in the presence of a palladium catalyst.
It can be used to catalyze ethynylation of quinolines and isoquinolines using calcium carbide in aqueous N,N-dimethylacetamide.
기타 정보
Reagent for the cleavage of silyl ethers and other silyl protecting groups; Catalyst for various reactions with silicon compounds; Use as a base in organic synthesis; Instability of anhydrous TBAF
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Chronic 3 - Eye Irrit. 2 - Repr. 2 - Skin Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
이미 열람한 고객
Tetrabutylammonium fluoride-induced dehydrobromination of vinyl bromides to terminal acetylenes
Okutani M and Mori Y
Tetrahedron Letters, 48(39), 6856-6859 (2007)
T.W. Greene et al.
Protective Groups in Organic Synthesis (1991)
An Efficient Method for the Production of Terminal Alkynes from 1, 1-Dibromo-1-alkenes and its Application in the Total Synthesis of Natural Product Dihydroxerulin
Liu S, et al.
advanced synthesis and catalysis, 357(2-3), 553-560 (2015)
An Efficient Method for the Production of Terminal Alkynes from 1, 1-Dibromo-1-alkenes and its Application in the Total Synthesis of Natural Product Dihydroxerulin
Liu S, et al.
Advanced Synthesis & Catalysis, 357(2-3), 553-560 (2015)
G. Majetich et al.
The Journal of Organic Chemistry, 51, 1745-1745 (1986)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.