추천 제품
분석
98%
형태
liquid
refractive index
n20/D 1.477 (lit.)
bp
167-169 °C (lit.)
density
0.931 g/mL at 25 °C (lit.)
SMILES string
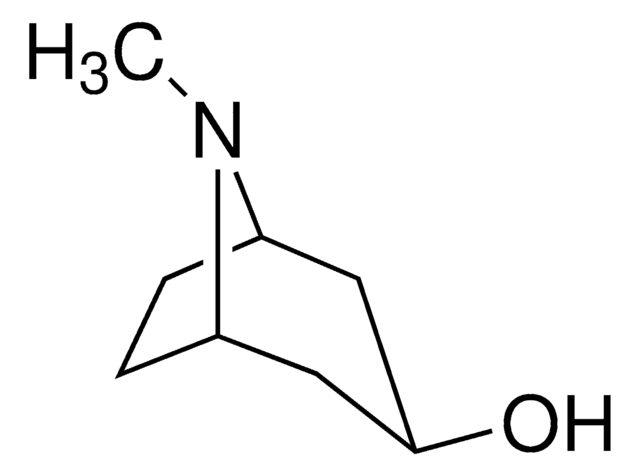
CN1[C@H]2CCC[C@@H]1CC2
InChI
1S/C8H15N/c1-9-7-3-2-4-8(9)6-5-7/h7-8H,2-6H2,1H3/t7-,8+
InChI key
XLRPYZSEQKXZAA-OCAPTIKFSA-N
일반 설명
Tropane (8-methyl-8-azabicyclo[3.2.1]-octane) is a bridged bicyclic N-containing compound. The 8-methyl-8-azabicyclo[3.2.1]-octane has a tropane ring system, which is an important substructure in a number of natural products and synthetic compounds of biological and medicinal importance. Production of tropane alkaloid in callus and root cultures of seven Hyoscyamus species has been studied. Tropane derivatives are reported to undergo rapid N-methyl inversion.
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point (°F)
107.6 °F - closed cup
Flash Point (°C)
42 °C - closed cup
개인 보호 장비
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
Abigail Moreno-Pedraza et al.
Metabolites, 9(7) (2019-07-07)
different Solanaceae and Erythroxylaceae species produce tropane alkaloids. These alkaloids are the starting material in the production of different pharmaceuticals. The commercial demand for tropane alkaloids is covered by extracting them from cultivated plants. Datura stramonium is cultivated under greenhouse
Lu Wang et al.
Angewandte Chemie (International ed. in English), 53(22), 5657-5661 (2014-04-18)
A palladium-catalyzed oxidative carbonylative esterification of a variety of functionalized alcohols under base- and ligand-free conditions has been demonstrated. A CO/olefin combination was utilized as the acylating reagent with O2 as a benign oxidant. Notably, the scope of the substrate
Jelena Dinic et al.
Current pharmaceutical design, 21(38), 5589-5604 (2015-10-03)
Resistance to chemotherapeutic drugs is one of the main obstacles to effective cancer treatment. Multidrug resistance (MDR) is defined as resistance to structurally and/or functionally unrelated drugs, and has been extensively investigated for the last three decades. There are two
Tehetena Mesganaw et al.
Organic process research & development, 18(9), 1097-1104 (2014-10-08)
A Rh-catalyzed C-H bond activation/alkenylation/electrocyclization cascade reaction provides diverse 1,2-dihydropyridines from simple and readily available precursors. The reaction can be carried out at low (<1%) Rh-catalyst loadings, and the use of the robust, air-stable Rh precatalyst, [RhCl(cod)]
Enhancing Tropane Alkaloid Production Based on the Functional Identification of Tropine-Forming Reductase in
Kaihui Zhao et al.
Frontiers in plant science, 8, 1745-1745 (2017-11-01)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.





![9-Azabicyclo[3.3.1]nonane N-oxyl 95%](/deepweb/assets/sigmaaldrich/product/structures/287/155/e2f4a2e1-1d4e-4bed-9187-9e16d23cbbbf/640/e2f4a2e1-1d4e-4bed-9187-9e16d23cbbbf.png)