추천 제품
분석
99%
광학 활성
[α]25/D +16°, c = 1 in 1 M HCl
mp
148-152 °C (lit.)
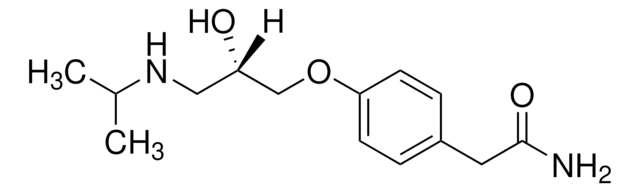
SMILES string
CC(C)NC[C@@H](O)COc1ccc(CC(N)=O)cc1
InChI
1S/C14H22N2O3/c1-10(2)16-8-12(17)9-19-13-5-3-11(4-6-13)7-14(15)18/h3-6,10,12,16-17H,7-9H2,1-2H3,(H2,15,18)/t12-/m1/s1
InChI key
METKIMKYRPQLGS-GFCCVEGCSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
이미 열람한 고객
Joni Agustian et al.
Chirality, 29(12), 847-853 (2017-10-01)
Kinetic resolution of (R,S)-atenolol is a faster strategy to produce (S)-atenolol. Since this racemate is a less soluble compound, resolution of its ester offers high concentrations in the process. A good analytical method is required to observe the enantiomer concentrations.
Joni Agustian et al.
Chirality, 29(7), 376-385 (2017-04-26)
As the (R)-enantiomer of racemic atenolol has no β-blocking activity and no lack of side effects, switching from the racemate to the (S)-atenolol is more favorable. Transesterification of racemic atenolol using free enzymes investigated as a resource to resolve the
Kevin F Morris et al.
Chemical physics, 457, 133-146 (2015-08-11)
Molecular dynamics simulations and NMR spectroscopy were used to compare the binding of two β-blocker drugs to the chiral molecular micelle poly-(sodium undecyl-(L)-leucine-valine). The molecular micelle is used as a chiral selector in capillary electrophoresis. This study is part of
Ulisse Garbin et al.
Mediators of inflammation, 2008, 367590-367590 (2008-04-26)
The endothelium plays a key role in the development of atherogenesis and its inflammatory and proliferative status influences the progression of atherosclerosis. The aim of this study is to compare the effects of two beta blockers such as nebivolol and
Chromatograms
application for HPLC자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.