718742
BrettPhos
98%
Synonym(s):
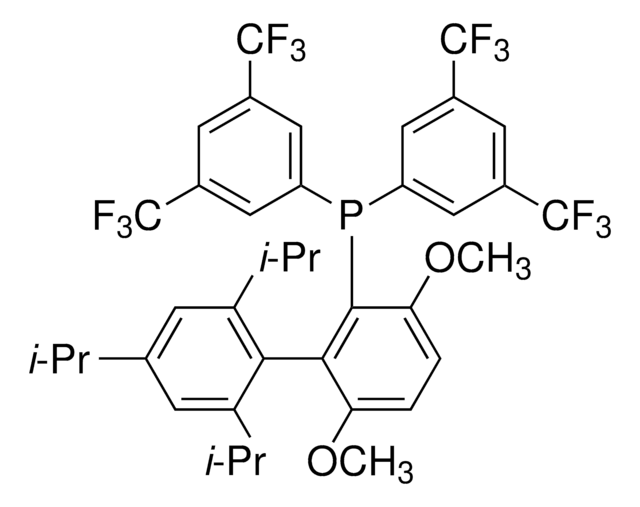
2-(Dicyclohexylphosphino)3,6-dimethoxy-2′,4′,6′-triisopropyl-1,1′-biphenyl
About This Item
Recommended Products
Quality Level
Assay
98%
form
solid
reaction suitability
reaction type: Cross Couplings
reagent type: ligand
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reagent type: ligand
reaction type: Fluorinations
greener alternative product score
old score: 8
new score: 1
Find out more about DOZN™ Scoring
greener alternative product characteristics
Waste Prevention
Atom Economy
Design for Energy Efficiency
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
mp
187-195 °C
functional group
phosphine
greener alternative category
SMILES string
COc1c(P(C2CCCCC2)C3CCCCC3)c(c4c(C(C)C)cc(C(C)C)cc4C(C)C)c(OC)cc1
InChI
1S/C35H53O2P/c1-23(2)26-21-29(24(3)4)33(30(22-26)25(5)6)34-31(36-7)19-20-32(37-8)35(34)38(27-15-11-9-12-16-27)28-17-13-10-14-18-28/h19-25,27-28H,9-18H2,1-8H3
InChI key
WDVGNXKCFBOKDF-UHFFFAOYSA-N
General description
Application
It can be used in:
- palladium-catalyzed trifluoromethylation of aryl chlorides
- Buchwald-Hartwig amination
- synthesis of 4-aryl and alkyl substituted, N6-alkylated pyridazine-3,6-diamines via a Buchwald protocol
Features and Benefits
- White crystalline solid
- Air- and moisture-stable
- Thermally stable
- Highly efficient
- Wide functional group tolerance
- Excellent selectivity and conversion
Storage Class Code
11 - Combustible Solids
WGK
nwg
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Related Content
An organic reaction toolbox provides structure-activity relationships for unique chemical reactions to optimally design and control small molecule synthesis.
The Buchwald group has developed a series of highly active and versatile palladium precatalysts and biarylphosphine ligands used in cross-coupling reactions for the formation of C-C, C–N, C–O, C–F, C–CF3, and C–S bonds. The ligands are electron-rich, and highly tunable to provide catalyst systems with a diverse scope, high stability and reactivity. Furthermore, the new series of precatalysts are air-, moisture and thermally-stable and display good solubility in common organic solvents. The use of precatalysts ensures the efficient generation of the active catalytic species and allows one to accurately adjust the ligand:palladium ratio. The ligands, precatalysts and methodology developed in the Buchwald group are user friendly and have rendered previously difficult cross couplings reactions, much easier to achieve.
Explore reliable, premium grade catalysis materials for your pharma or industrial project. Specialty chemicals and formulations are available in bulk quantities and volumes from a few grams to multi-metric tons with complete documentation to simplify your leap from development to commercialization.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service










![[2-(Dicyclohexylphosphino)-3,6-dimethoxy-2′,4′,6′-triisopropyl-1,1′-biphenyl]gold(I) bis(trifluoromethanesulfonyl)imide](/deepweb/assets/sigmaaldrich/product/structures/361/949/e30e9505-889a-4ffd-9c57-f66a0a20b299/640/e30e9505-889a-4ffd-9c57-f66a0a20b299.png)


