1067704
USP
Betamethasone dipropionate
United States Pharmacopeia (USP) Reference Standard
Synonym(s):
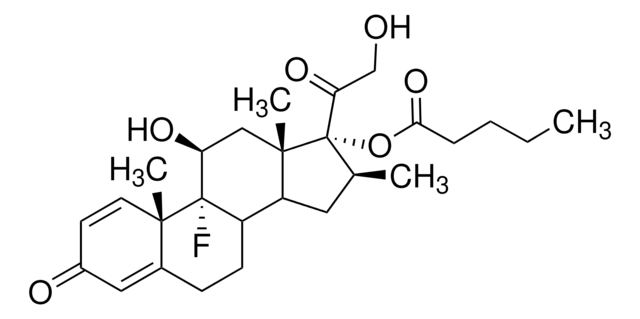
Betamethasone 17,21-dipropionate, 9α-Fluoro-16β-methyl-11β,17α,21-trihydroxy-1,4-pregnadiene-3,20-dione 17,21-dipropionate
About This Item
Recommended Products
grade
pharmaceutical primary standard
API family
betamethasone
manufacturer/tradename
USP
application(s)
pharmaceutical (small molecule)
format
neat
SMILES string
[H][C@@]12C[C@H](C)[C@](OC(=O)CCl)(C(=O)COC(=O)CC)[C@@]1(C)C[C@H](O)C3(F)[C@@]2([H])CCC4=CC(=O)C=C[C@]34C
InChI
1S/C27H34ClFO7/c1-5-22(33)35-14-21(32)27(36-23(34)13-28)15(2)10-19-18-7-6-16-11-17(30)8-9-24(16,3)26(18,29)20(31)12-25(19,27)4/h8-9,11,15,18-20,31H,5-7,10,12-14H2,1-4H3/t15-,18-,19-,20-,24-,25-,26?,27-/m0/s1
InChI key
UOCNBCVPZWLUKX-HOBFILNXSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- Efficacy in Alopecia Areata Treatment: A study compared the effectiveness of topical betamethasone dipropionate with minoxidil in treating alopecia areata, highlighting its potent anti-inflammatory properties and its role in promoting hair regrowth. This positions betamethasone dipropionate as a key component in dermatological research aimed at understanding and improving treatment modalities for hair loss conditions (Aslam et al., 2024).
- Post-Surgical Pain Management: Research demonstrated the utility of a cocktail containing betamethasone dipropionate in reducing pain and prolonging analgesic effects post-total knee arthroplasty. This study emphasizes the pharmacokinetic benefits of betamethasone dipropionate in clinical pain management, providing valuable insights for ongoing pharmacological innovations (Luo et al., 2024).
- Improvement in Plaque Psoriasis: Betamethasone dipropionate was analyzed for its effectiveness in treating plaque psoriasis when used as part of a calcipotriol/betamethasone aerosol foam. The results underscore its significant role as a glucocorticoid receptor agonist, enhancing therapeutic outcomes for patients with psoriasis, thereby solidifying its place in anti-inflammatory steroid studies and psoriasis treatment compounds (Gerdes et al., 2024).
Biochem/physiol Actions
Analysis Note
Other Notes
related product
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Repr. 1B - STOT RE 2
Target Organs
Liver,Kidney,Endocrine system
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service