45520
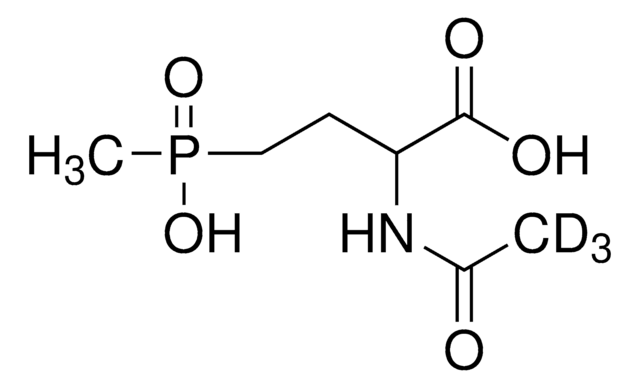
Glufosinate-ammonium
PESTANAL®, analytical standard
Synonym(s):
2-Amino-4-(hydroxymethylphosphinyl)butyric acid ammonium salt, DL-Phosphinothricin
About This Item
Recommended Products
grade
analytical standard
Quality Level
product line
PESTANAL®
shelf life
limited shelf life, expiry date on the label
technique(s)
HPLC: suitable
gas chromatography (GC): suitable
mp
215 °C
application(s)
agriculture
environmental
format
neat
SMILES string
N.CP(O)(=O)CCC(N)C(O)=O
InChI
1S/C5H12NO4P.H3N/c1-11(9,10)3-2-4(6)5(7)8;/h4H,2-3,6H2,1H3,(H,7,8)(H,9,10);1H3
InChI key
ZBMRKNMTMPPMMK-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- Human serum samples by mixed-mode solid-phase extraction (SPE), tert-butyldimethylsilyl (t-BDMS) derivatization and gas chromatography-mass spectrometry (GC-MS) operating on electron impact (EI) mode of detection.
- Water samples by ion-exchange cleanup and derivatization followed by analysis using GC coupled to tandem mass spectrometry (MS/MS) equipped with EI mode of detection.
- Finnish arable soils by high performance liquid chromatography combined with fluorescence detection (HPLC-FLD).
Legal Information
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Repr. 1B - STOT RE 2
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Glyphosate and related compounds are measured in oatmeal and infant cereal using ion-exchange polymer-based particles for HPLC and SPE. Low level detection was obtained.
Glyphosate analysis: LC-MS/MS method with Supel™ Carbon LC U/HPLC column for stability and retention.
Glyphosate analysis: LC-MS/MS method with Supel™ Carbon LC U/HPLC column for stability and retention.
Glyphosate analysis: LC-MS/MS method with Supel™ Carbon LC U/HPLC column for stability and retention.
Protocols
Protocol for LC/MS Analysis of Glyphosate and Metabolites on apHera™ NH2, 2 mm I.D. Column
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service