A2636
S-(2-Aminoethyl)-L-cysteine hydrochloride
≥98% (TLC)
Synonym(s):
L-4-Thialysine hydrochloride
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C5H12N2O2S · HCl
CAS Number:
Molecular Weight:
200.69
Beilstein:
3697262
EC Number:
MDL number:
UNSPSC Code:
12352209
eCl@ss:
32160406
PubChem Substance ID:
NACRES:
NA.26
Recommended Products
Product Name
S-(2-Aminoethyl)-L-cysteine hydrochloride, ≥98% (TLC)
Quality Level
Assay
≥98% (TLC)
form
powder
color
white to off-white
storage temp.
2-8°C
SMILES string
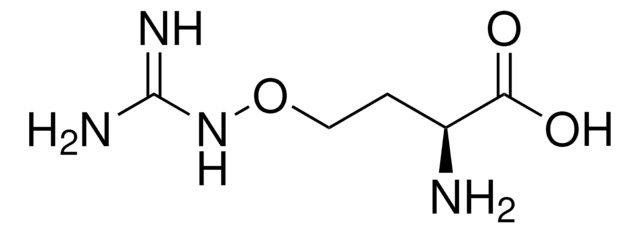
Cl.NCCSC[C@H](N)C(O)=O
InChI
1S/C5H12N2O2S.ClH/c6-1-2-10-3-4(7)5(8)9;/h4H,1-3,6-7H2,(H,8,9);1H/t4-;/m0./s1
InChI key
CVHKULVNPGAEQM-WCCKRBBISA-N
Looking for similar products? Visit Product Comparison Guide
Biochem/physiol Actions
S-(2-Aminoethyl)-L-cysteine (AEC) hydrochloride is used as a lysine analogue for comparative analysis with other lysine analogues. S-(2-Aminoethyl)-L-cysteine is an alternative substrate useful for characterizing lysine cyclodeaminase from Streptomyces pristinaespiralis. AEC may be used as a non-antibiotic selection agent for genetically engineered soybeans expressing a lysine insensitive DHPS gene. AEC is being studied as an amino acid antibiotic.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Ulf Göransson et al.
Analytical biochemistry, 318(1), 107-117 (2003-06-05)
The expression of cyclotides-macrocyclic plant peptides-was profiled in six violets, Viola cotyledon, V. biflora, V. arvensis, V. tricolor, V. riviniana, and V. odorata, by LC-MS. All were found to express notably complex mixtures, with single species containing >50 cyclotides. To
Do Youn Jun et al.
Biochemical pharmacology, 66(12), 2291-2300 (2003-11-26)
We first report the mechanism for the inhibitory effect of the lysine analog, thialysine on human acute leukemia Jurkat T cells. When Jurkat T cells were treated with thialysine (0.32-2.5 mM), apoptotic cell death along with several biochemical events such
Asri Peni Wulandari et al.
FEBS letters, 522(1-3), 35-40 (2002-07-04)
In Thermus thermophilus homocitrate synthase (HCS) catalyzes the initial reaction of lysine biosynthesis through alpha-aminoadipic acid, synthesis of homocitrate from 2-oxoglutarate and acetyl-CoA. HCS is strongly inhibited by lysine, indicating that the biosynthesis is regulated by the endproduct at the
Dasantila Golemi-Kotra et al.
The Journal of biological chemistry, 279(33), 34665-34673 (2004-05-21)
Beta-lactamases and penicillin-binding proteins are bacterial enzymes involved in antibiotic resistance to beta-lactam antibiotics and biosynthetic assembly of cell wall, respectively. Members of these large families of enzymes all experience acylation by their respective substrates at an active site serine
M N Cahyanto et al.
Journal of applied microbiology, 102(3), 674-679 (2007-02-21)
To enhance L-lysine secretion in Lactobacillus plantarum. An S-2-aminoethyl-L-cystein (AEC)-resistant mutant of L. plantarum was isolated, and it produced L-lysine at considerably higher level than the parent strain. Aspartokinase in the mutant has been desensitized to feedback inhibition by L-lysine.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service