Glioma Markers for High-Grade Gliomas and Low-Grade Gliomas
Gliomas are one of the most aggressive and lethal solid tumors of the central nervous system. Despite the alarming numbers, the molecular pathogenetic mechanisms of gliomas are not yet fully elucidated. Nowadays, several approaches aim to understand the biology of the disease and identify promising markers, which may provide effective novel therapies. This article summarizes some developments in glioma classification and the key molecular markers for glioma stratification, highlights the glioma proteome through a list of relevant genes with favorable and unfavorable prognostic values in glioma, and focuses on the glioma tumor microenvironment that may provide helpful insights when developing novel therapeutic strategies.
- What Are Gliomas?
- Glioma vs. Glioblastoma
- Current Landscape of Glioma Treatments
- Glioma Classifications
- Molecular Pathology of Gliomas
- Distinctive Features: Adult vs Pediatric Gliomas
- The Glioma Proteome
- The Glioma Tumor Microenvironment
- Glioma TME Markers
- Reliable Antibodies for Targeting Glioma Markers
What Are Gliomas?
Gliomas, commonly known as brain tumors, are solid tumors of the central nervous system (CNS). They arise from the neoplastic transformation of glial cells, namely astrocytes, oligodendrocytes, and ependymal cells.
Brain tumors comprise approximately 2% of all adult cancers but form a larger fraction within the group of childhood tumors. Gliomas account for about 80% of all malignant brain tumors and are classified according to the cell type of origin, differentiation, and malignancy grade. Gliomas show considerable variability in age of onset, grade of severity, histological features, and ability to progress, as well as to metastasize.
Survival time after diagnosis with glioma varies significantly depending on grade. According to the World Health Organization (WHO) classification, gliomas are divided into four grades. Grade 1, 2, and 3 are classified as low-grade gliomas, while grade 4 are classified as high-grade gliomas. Unfortunately, the prognosis for high-grade gliomas is poor due to limited possibilities of curative treatment.
Glioma vs. Glioblastoma
The most generic form of glioma is an astrocytoma, representing approximately 50% of all gliomas. Grade 4 astrocytoma is also known as glioblastoma or glioblastoma multiforme (GBM).
GBM is the most frequent and malignant histological type, accounting for 65% of gliomas with very poor 5-year survival (less than 5%). GBM is typically classified into three subtypes, namely pro-neural, classical, and mesenchymal, according to the gene expression of various biomarkers, including platelet-derived growth factor receptor (PDGFR), neurofilament light chain (NF-L), epidermal growth factor receptor (EGFR), and CD44, respectively. Pro-neural to mesenchymal transition of glioma is associated with aggressive phenotypes, unfavorable prognosis, and treatment resistance.
Current Landscape of Glioma Treatments
Gliomas are well-known tumors at the molecular level. However, these advances have not been translated into therapeutic benefits. While our knowledge about the molecular biology of gliomas is rapidly expanding and is, to some extent, already assisting us in the design of tumor-tailored therapeutics, such as immunotherapy treatments, we are still struggling to develop efficient treatments. As a result, the overall survival rate of gliomas has not significantly increased in the last decades.
Typical treatments for gliomas involve surgical resection of the tumor mass, radiotherapy, and chemotherapy treatments. However, relapse or even recurrence of gliomas is common and mainly due to their infiltrative growth and their high proliferative abilities. Due to the absence of definitive surgical and medical treatments currently available, an early diagnosis coupled with an accurate tumor classification is crucial to select a personalized treatment.
Moreover, the functional heterogeneity in gliomas is defined not only by the genetic makeup of glioma cells but also through microenvironment-driven epigenetic influences that regulate glioma cell stemness. Hence, elucidating the state transition programs and mechanisms driving cellular plasticity is essential to overcome current therapeutic limitations in GBM.
We must expand our knowledge as a step toward the design of practical and safe treatments. Therefore, the identification of molecular biomarkers is unquestionably essential and urgent for an accurate prognosis and development of critical therapeutic targets in gliomas.
Glioma Classifications
Since 2008 the classification of brain tumors by the WHO has continued to evolve (WHO CNS5, 2021).1 The 5th edition classification emphasizes the need for a classification of gliomas based on histological and molecular genetic features for clinical diagnosis and outcome prediction. With the WHO CNS5 2021 edition, several IHC diagnostic and prognostic markers have been added to the diagnostic criteria of gliomas. The new seven-layers approach for glioma classification is shown below.
Gliomas: The 7 Layers Classification (WHO 2021)1
- Isocitrate dehydrogenase (IDH1) mutations
- a-thalassemia/mental-retardation-syndromeX-linked gene (ATRX) expression
- 1p/19q co-deletion
- CDKN2A/B homozygous deletion on 9p21
- TERT promoter mutation/EGFR gene amplification and/or chromosomes 7 gain and 10 loss (+7/–10)
- Histone H3 G34R/V mutations
- Histone H3 K27M/ mutations
Molecular Pathology of Gliomas
Antibodies against ATRX, the mutated forms of IDH1 (IDH R132H), and histone H3 (H3K27M) are used to detect the respective mutations. Analysis of deletion of 1p and 19q chromosome arms is done by multiplex ligation-dependent probe amplification (MLPA) or, less commonly, by fluorescent in situ hybridization. The distinction between the different forms of glioma is mainly based on morphological features, mutations, and chromosomal aberrations as shown below.
Molecular Pathology in High-Grade Gliomas
- Methylation: MGMT, PTEN, RB1, TP53, CDKN2A, PDGFB, EMP3, SOCS1, PCDHGA11, OLIIG1/2
- Chromosomal aberrations:
- Gain: 1q, 199, 20q
- Loss: 6q, 9p, 139, 14q, 229
- Concomitant: +7/-10
- Mutations: PTEN, ATRX, TP53, RB1, IDH1/2, NF1, EGFR
- Amplifications: MET, EGFR, PIK3CA, PDGFRA, CCND2, MDM2/4
- Deletions: CDKN2A, CDKN2B, CDKN2C, PTEN, RB1, NFKB1A
In neuropathological diagnostics of gliomas, antibodies directed towards proteins such as IDH (isocitrate dehydrogenase), ATRX (alpha thalassemia/mental retardation syndrome X-linked), GFAP (glial fibrillary acidic protein), SYN (synaptophysin), EGFR (epidermal growth factor receptor), p53 (tumor suppressor protein 53), and the proliferation marker Ki-67 (MKI67) are the gold standard and routinely used.
Immunohistochemistry (IHC) plays a vital role in distinguishing between different tumor types. Figures 1 and 2 show some examples of IDH1, ATRX, and GFAP multiplexed IHC-IF staining in different glioma grades.
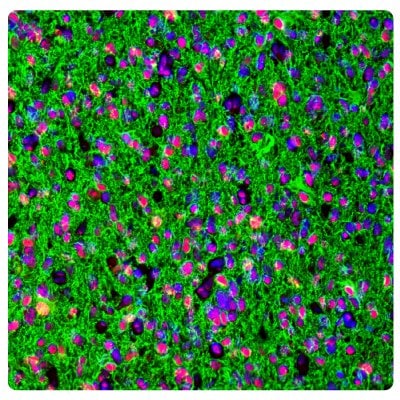
Figure 1A.ATRX and GFAP in astrocytoma. Multiplexed IHC-IF staining of astrocytoma showing ATRX (nuclear, in red) and GFAP (cytoplasmic, in green) immunoreactivity in tumor cells using Anti-ATRX antibody and Anti-GFAP antibody. Nuclei were counterstained with DAPI (in blue).

Figure 1B.ATRX and IDH1 in oligodendrocytoma. Multiplexed IHC-IF staining of anaplastic oligodendroglioma showing ATRX (nuclear, in red) and IDH1 (cytoplasmic, in green) immunoreactivity in tumor cells using Anti-ATRX antibody and Anti-IDH1 antibody. Nuclei were counterstained with DAPI (in blue).

Figure 2A.ATRX and GFAP in glioblastoma. Multiplexed IHC-IF staining of glioblastoma multiforme showing ATRX (nuclear, in red) and GFAP (cytoplasmic, in green) immunoreactivity in tumor cells using Anti-ATRX antibody and Anti-GFAP antibody. Nuclei were counterstained with DAPI (in blue).

Figure 2B.ATRX and IDH1 in glioblastoma. Multiplexed IHC-IF staining of glioblastoma multiforme showing ATRX (nuclear, in red) and IDH1 (cytoplasmic, in green) immunoreactivity in tumor cells using Anti-ATRX antibody and Anti-IDH1 antibody. Nuclei were counterstained with DAPI (in blue).
Distinctive Features: Adult vs Pediatric Gliomas
An essential update in the WHO CNS5 2021 classification of brain tumors is the distinction of diffuse gliomas into adult-type and pediatric-type gliomas, all with distinct responses to treatment and outcomes.
The distinction between different forms of gliomas is mainly based on morphological features; however, IHC analysis plays a vital role in distinguishing between different tumor types, particularly when the tumor is poorly differentiated. Gliomas are classified according to the cell type of origin, differentiation, and malignancy grade. The type, grade, and molecular signatures of glioma determine diagnosis and treatment options. Based on integrative molecular profiling, diffusely infiltrating gliomas in adults are separated into three overarching groups depending on origins, mutations in isocitrate dehydrogenase (IDH), ATRX, and 1p/19q expression. In the pediatric population, diffuse gliomas are classified as low-grade gliomas and high-grade gliomas.
Below describes the classification, grade, and key diagnostic genes of adult- and pediatric-type of diffuse gliomas.
Adult-Type Diffuse Gliomas
- Astrocytomas
- IDH-mutant, 1p/19q non-co-deleted Grade 2,3,4 IDH1-2, ATRX, TP53, CDKN2A/B
- Oligodendrogliomas
- IDH-mutant, 1p/19q-co-deleted Grade 2,3 IDH1-2, 1p/19q, TERT, CIC, FUBP1, NOTCH1
- Glioblastomas
- IDH-wildtype Grade 4 TERT, chromosomes +7/-10, EGFR
Pediatric-Type Diffuse Gliomas
- Low-Grade
- Diffuse Astrocytoma
- Grade 1
- MYB- or MYBL1-altered
- Angiocentric Glioma
- Grade 1
- ATRX, p53, IDH1 (R132H), BRAF V600E, H3 K27M
- Polymorphous Neuroepithelial Tumor of the Young
- Grade 1
- FGFR3 amplification, BRAF V600E
- Diffuse Low-Grade Glioma
- G*
- МАРК
- Diffuse Astrocytoma
- High-Grade
- Diffuse Midline Glioma
- G*
- H3 K27-altered, PDGFR-a
- Diffuse Hemispheric Glioma
- Grade 4
- H3 G34-mutant
- Diffuse Pediatric-Type High-Grade Glioma
- G*
- H3-wildtype and IDH-wildtype
- Infant-Type Hemispheric Glioma
- G*
- ALK, ROS1, NTRK1/2/3, or MET
- Diffuse Midline Glioma
G* = attribution of a precise grade depends on the specific characterization following the integration of histopathological and molecular information
Numerous reports have highlighted substantial differences in adult and pediatric gliomas mainly based on histological observations, frequency, location, and pathologic spectrum.2 Pediatric high-grade gliomas, for example, often arise in brain regions that are rarely targeted in adult disease.
Pediatric diffuse gliomas are more complex and molecularly distinct from those in adults. Pediatric gliomas are generally classified as low-grade and high-grade and are characterized by circumscribed growth and frequent H3 and BRAF gene fusions or mutations.1
On the other hand, PTEN mutations and EGFR amplification, frequent in adult primary glioblastoma, are less common in children.3 Furthermore, in adults, secondary glioblastomas rarely contain EGFR amplification.4
Mutations in TP53, CDKN2A, and PIK3CA are common in adult and pediatric high-grade gliomas.5 Furthermore, the platelet-derived growth factor receptor-α (PDGFR-α) is the predominant target of focal amplification in high-grade childhood glioma and may be a valuable target for the pediatric population.6
Significant differences in copy number alterations distinguish childhood and adult glioblastoma. No IDH1 hotspot mutations were found in pediatric tumors, highlighting molecular differences with adult secondary glioblastoma. Frequent gain of chromosome 1q and lower frequency of chromosome 7 gain and 10q loss also clearly distinguished childhood from adult high-grade gliomas.6
The Glioma Proteome
The glioma proteome is revealed and accessible on the Human Protein Atlas website. It is built on the GBM data available from The Cancer Genome Atlas program (TCGA) and combines transcriptomic data with antibody-based protein data.
The transcriptome analysis shows that 72% (n=14,370) of all human genes (n=20,090) are expressed in glioma. It also shows that 895 genes have elevated expression in glioma, 267 of which are suggested as prognostic. Among the prognostic genes, 200 are associated with unfavorable prognosis and 67 are associated with favorable prognosis.
The top 20 significant genes related to unfavorable and favorable prognosis in GBM are listed in Table 1 and Table 2, respectively. For unfavorable genes, higher relative expression levels at diagnosis give significantly lower overall survival rates. For favorable genes, higher relative expression levels at diagnosis give significantly higher overall survival rates.
Figure 3 shows the different compositions of mutated genes across high-grade and low-grade gliomas.
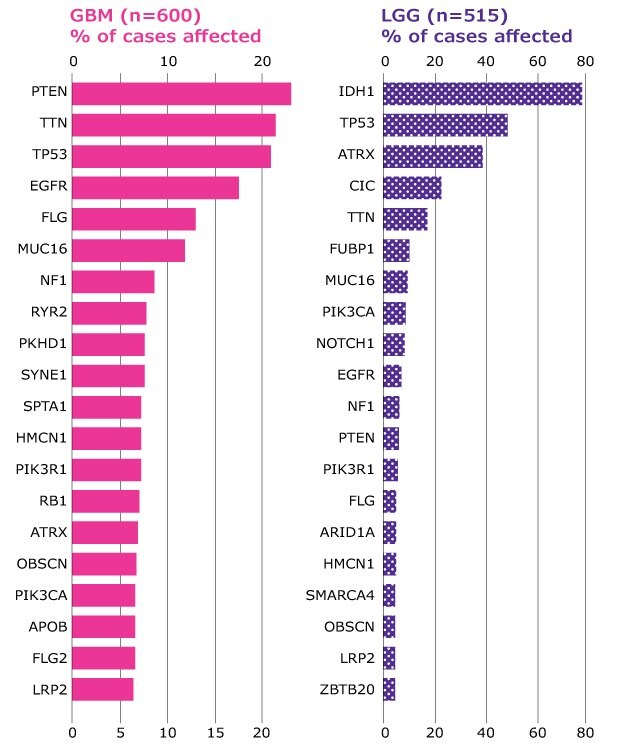
Figure 3.High-grade and low-grade gliomas differ in the composition of mutated genes. Distribution of most frequently mutated genes in high-grade (GBM) and low-grade gliomas (LGG) expressed as % of total cases (n). Source TCGA.
The Glioma Tumor Microenvironment
The dynamic communication between tumor cells and the surrounding glioma tumor microenvironment (TME) plays a crucial role in the sustained growth, proliferation, and invasion of gliomas, survival, evasion of cell death, metabolism, migration, and metastasis.
The glioma TME is highly heterogeneous, consisting of a multiplex of both cancerous and non-cancerous cells, including endothelial cells (ECs), immune cells, glioma stem-like cells (GSCs), and astrocytes, as well as noncellular components such as the extracellular matrix (ECM).7,8
Several means of intercellular communication between the glioma tumoral cells and the TME have been documented. In the glioma TME, cells communicate through endothelial cells, growth factors, cytokines, chemokines, monocytes, macrophages, mast cells, microglia, T-cells, astrocytes, oligodendrocytes, and cancer stem cells.9,10
All the different means of communication show a multifaceted role in supporting several hallmarks of cancer. Understanding the dynamic glioma tumor microenvironment is necessary for the development of new immunotherapeutic strategies. Glioma cells coexist with normal non-neoplastic cells. Glioma cells proliferate in a hypoxic immunosuppressive TME, with aberrant vasculature, abundant and distinct extracellular matrix, and impaired blood-brain tumor barrier.
Four mechanisms in the TME have emerged as paramount for our understanding of gliomas and crucial for the development of new treatment strategies such as immunomodulation, angiogenesis, cancer stem cells, and drug resistance. Many of these mechanisms are interdependent and carry specific molecular signatures.
Immunomodulation
In recent years, immunotherapy has become one of the major options for anti-cancer therapy. However, the complex mechanisms of glioma immunogenicity and microenvironment interactions have only partially been elucidated.11 The ability of tumor cells to suppress both local and systemic immune responses and to hijack the communication with the TME, severely restricts treatment efficacy.
Immune cells constitute an important component of the glioma TME as they can reach up to 50% of the tumor mass content. Immune cells in glioma switch on the inflammatory process in the TME thus enhancing tumor development.12,13 Consistently, high-grade gliomas have a more immunosuppressive profile than low-grade gliomas.
Among the immune-related genes ADORA2A, CD160, CD276, NRP1, and VTCN1 are significantly overexpressed in low-grade gliomas. In high-grade gliomas, VTCN1, BTNL2, and METTL21B are overexpressed, while the expression of CD86, HAVCR2, LAIR1, and VSIR is significantly decreased.
Cytokines and chemokines secreted by glioma cells induce infiltration of immunosuppressive cells (MDSCs, Tregs, and TAMs) and the acquisition of pro-tumoral phenotypes by myeloid cells (M2 phenotype differentiation and PDL-1 and B7 expression).
Angiogenesis
The poor prognosis for glioblastoma is also partly due to the lack of successful drug delivery across the blood-brain tumor barrier. Glioblastomas are highly vascularized tumors, and glioma growth depends on the formation of new blood vessels. Angiogenesis is a complex process involving the proliferation, migration, and differentiation of vascular endothelial cells under the stimulation of specific signals.
The high metabolic demands of high-grade glioma, for instance, create hypoxic areas, that trigger the expression of PLVAP (a vascular marker of a disrupted blood-brain barrier) and angiogenesis, leading to the formation of abnormal vessels and a dysfunctional blood-brain tumor barrier. Endoglin (CD105), a marker for immature blood vessels, is significantly higher in glioblastoma compared with peritumoral normal tissue.14
Glioma Cancer Stem Cells and Drug Resistance
Chemoresistance and reoccurrence are major issues in the management of glioma and the main cause of mortality. The reoccurrence of gliomas is largely associated with the ability of tumor cells to regenerate from treatment-resistant cancer stem cells within and surrounding the primary tumor after initial treatment. Glioma cancer stem cells (GSCs) are the main drivers of uncontrolled cell growth in high-grade gliomas, but also the resistance to therapy.7,15
GSCs act through a core set of neurodevelopmental transcription factors and oncogenes. High-grade gliomas show elevated transcription of stem cells such as ALDH1A1, EZH2, GFAP, SALL4, NANOG, and POSTN. Molecular surface markers such as CD133, CD44, A2B5, CD15, and CD171 have also been implicated in the reoccurrence of gliomas and increased aggressiveness of glioblastoma.
An example is ChI3L1 as a modulator of stem cell cellular states. Phenotypic plasticity in human tumors can be driven by activation of the epithelial-to-mesenchymal transition (EMT) process by which cells acquire plasticity and gain the properties of stem cells. CHI3L1 is a secreted protein acting as a modulator of stem cell states and is highly expressed in gliomas. CHI3L1 is used as a marker for invasion, migration, and angiogenesis in glioblastoma.16 Figure 4 shows an example of immunostaining astrocytoma with an Anti-CHI3L1 antibody.

Figure 4A.Fluorescence immunohistochemistry images of human normal cortex tissue samples stained with the Anti-CHI3L1 antibody. Nuclei are counterstained by DAPI (in blue).
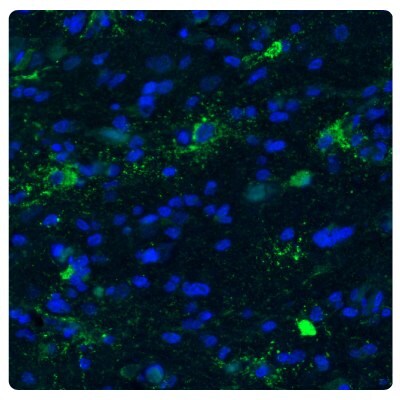
Figure 4B.Fluorescence immunohistochemistry images of astrocytoma samples stained with the Anti-CHI3L1 antibody showing strong protein expression (in green). Nuclei are counterstained by DAPI (in blue).
Glioma TME Markers
TME, including infiltration of myeloid cells such as microglia and macrophages, plays a significant role in glioma progression.17 Glioma-accumulated microglia/macrophages are a mixed cell population with pro- and anti-tumoral properties, although tumor-supporting effects generally predominate.
HLA-DRA
Microglial cells which present the antigens to the CD4-positive T-cells, express HLA-DRA protein which belongs to the HLA class II proteins.18 HLA expression is increased in glioblastoma, and there is some evidence suggesting that the HLA family can be used as a specific molecular target in the treatment of glioblastoma.19
METTL21B
METTL21B is involved in cell adhesion, angiogenesis, and cell proliferation. Its expression is positively associated with glioma grade at the protein level. METTL21B facilitates immune evasion of tumors and affects prognosis by mediating macrophage polarization from M1 to M2 and regulating the expression of immune checkpoints. Nevertheless, high METTL21B levels are associated with a better response to immune checkpoint blockade therapy. Because of its substrate specificity, METTL21B is a promising target for the treatment of glioma.20
Figure 5 shows the high METTL21B expression in glioblastoma tumor cells as well as an increased number of activated microglial cells (HLA-DRA) compared to controls.

Figure 5A.Multiplexed IHC-IF staining of human normal cortex samples using Anti-HLA-DRA monoclonal (cytoplasmic, in green) and Anti-METTL21B polyclonal (nuclear, in red) antibodies. Nuclei are counterstained with DAPI (in blue). White arrow indicates activated microglia (HLA-DRA positive staining).
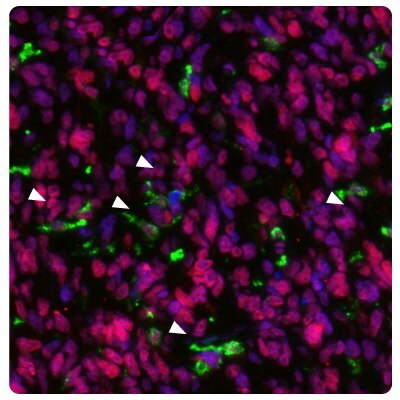
Figure 5B.Multiplexed IHC-IF staining of glioblastoma samples using Anti-HLA-DRA monoclonal (cytoplasmic, in green) and Anti-METTL21B polyclonal (nuclear, in red) antibodies. Nuclei are counterstained with DAPI (in blue). White arrows indicate activated microglia (HLA-DRA positive staining).
SALL4
Targeting GSCs is an extremely important aspect of the clinical treatment of gliomas. A better comprehension of glioma GSCs provides functional insights into the dynamics of cellular communication during glioma genesis, creating new opportunities for diagnostics and therapeutics.
The transcription factor SALL4 participates in cell proliferation, apoptosis, cycle, invasion, evolution, and drug resistance. SALL4 is overexpressed in glioma and correlated with poor outcomes.21 SALL4 acts by strengthening the PI3K/AKT signaling pathway (which is a well-known pathway in the regulation of tumorigenesis, significantly activated in glioma) thus reducing the expression of the tumor suppressor PTEN (Liu 2017).22
Figure 6 shows the characterization of SALL4 in human and mouse tissues and Figure 7 shows an example of SALL4 upregulation in glioma samples.
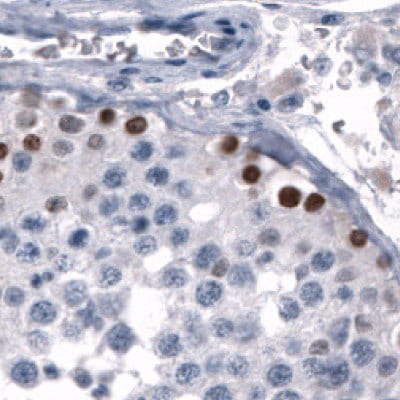
Figure 6A.Immunohistochemical staining using the Anti-SALL4 monoclonal antibody showing nuclear positivity in germinal cells in testis.
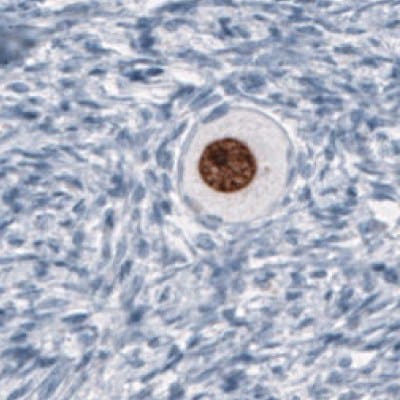
Figure 6B.Immunohistochemical staining using the Anti-SALL4 monoclonal antibody showing nuclear positivity in germinal cells in an oocyte.
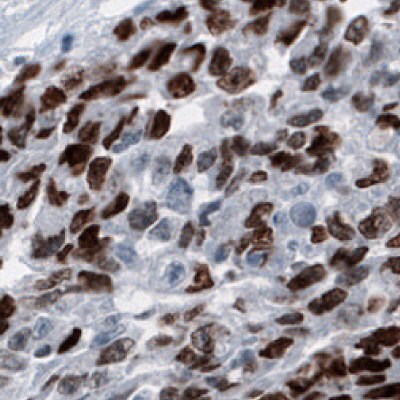
Figure 6C.Immunohistochemical staining using the Anti-SALL4 monoclonal antibody showing nuclear positivity in germinal cells in embryonal testis carcinoma human tissues.
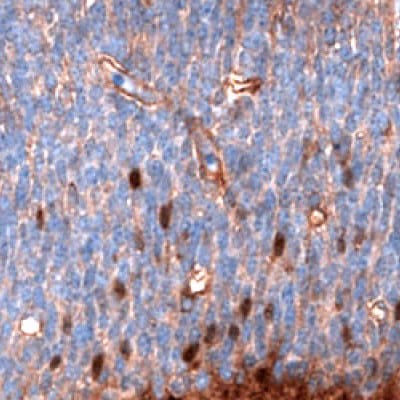
Figure 6D.Immunohistochemical staining using the Anti-SALL4 monoclonal antibody showing nuclear positivity in a subset of cells in the developing brain in mouse embryo E11.
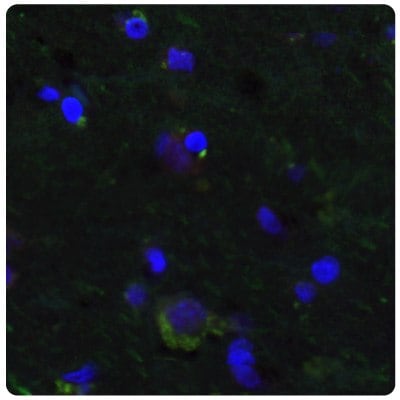
Figure 7A.Multiplexed IHC-IF staining of human normal cortex samples using the Anti-SALL4 monoclonal (nuclear, in red) and the Anti-PTEN monoclonal (cytoplasmic, in green) antibodies. Nuclei are counterstained with DAPI (in blue).
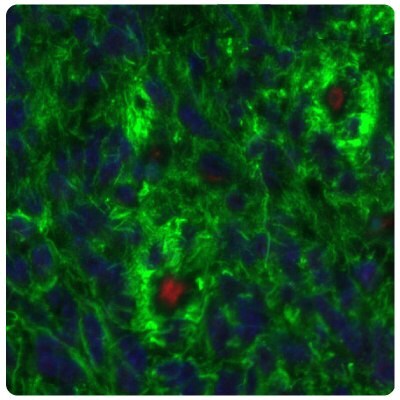
Figure 7B.SALL4 is upregulated in glioma samples compared to Figure 7A. Multiplexed IHC-IF staining of glioblastoma samples using the Anti-SALL4 monoclonal (nuclear, in red) and the Anti-PTEN monoclonal (cytoplasmic, in green) antibodies. Nuclei are counterstained with DAPI (in blue).
EZH2
The transcription factor EZH2 is highly expressed in gliomas and is a potential target for immune therapy. EZH2 is a histone H3 lysine methyltransferase that promotes tumorigenesis in a variety of human malignancies including gliomas by altering the expression of tumor suppressor genes. EZH2 overexpression in gliomas is associated with several immune checkpoints, cell cycle, DNA replication, mismatch repair, p53 signaling, and tumor-infiltrating lymphocytes.23
ALDH1A3
The aldehyde dehydrogenases ALDH1A3 are associated with cell adhesion and tumor invasion and are a marker of the Mes-subtype of gliomas. ALDH is a marker of cancer stem cells associated with the malignant phenotype in gliomas. Among the ALDH isoforms, ALDH1A3 is overexpressed in high-grade gliomas compared to low-grade gliomas, while ALDH1A1 is overexpressed in low-grade gliomas compared to high-grade gliomas. Most of the Mes-subtypes have high ALDH1A3 mRNA expression, indicating ALDH1A3 as a useful marker for the Mes-subtype of gliomas.24
Figure 8 shows immunostainings of EZH2, PARP1, and ALDH1A3 expression in various sample types.
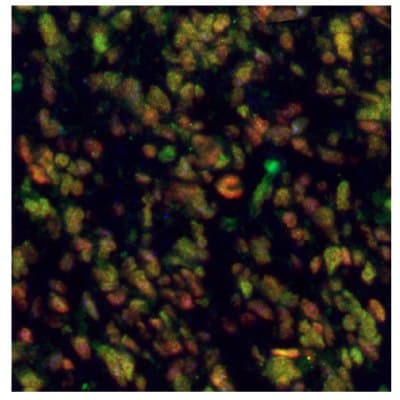
Figure 8A.Multiplexed IHC-IF staining of human glioblastoma using the Anti-EZH2 monoclonal (nuclear, in green), the Anti-PARP1 monoclonal (nuclear, in red), and the Anti-ALDH1A3 monoclonal (cytoplasmic, in blue) antibodies.
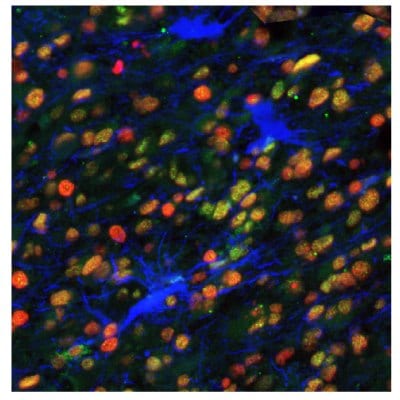
Figure 8B.Multiplexed IHC-IF staining of human astrocytoma samples using the Anti-EZH2 monoclonal (nuclear, in green), the Anti-PARP1 monoclonal (nuclear, in red), and the Anti-ALDH1A3 monoclonal (cytoplasmic, in blue) antibodies.
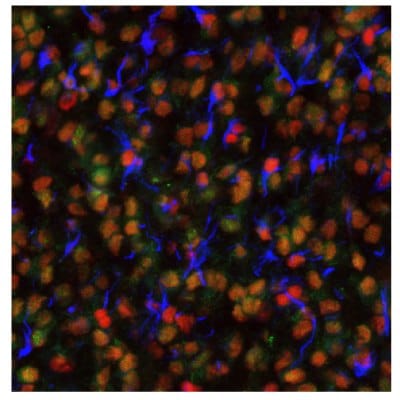
Figure 8C.Multiplexed IHC-IF staining of human oligodendrocytoma samples using the Anti-EZH2 monoclonal (nuclear, in green), the Anti-PARP1 monoclonal (nuclear, in red), and the Anti-ALDH1A3 monoclonal (cytoplasmic, in blue) antibodies.

Figure 8D.Multiplexed IHC-IF staining of human normal cortex samples using the Anti-EZH2 monoclonal (nuclear, in green), the Anti-PARP1 monoclonal (nuclear, in red), and the Anti-ALDH1A3 monoclonal (cytoplasmic, in blue) antibodies.
SGO2
Shugoshin 2 (SGO2) is critical in cell division and cell cycle progression. SGO2 plays a crucial role in glioma cell proliferation. Data show that SGO2 expression positively correlates with WHO grading of human gliomas.25
Figure 9 shows immunostainings of EZH2 and SGO2 expression in human normal cortex and glioblastoma samples.

Figure 9A.Multiplexed IHC-IF staining of human normal cortex samples using the Anti-EZH2 monoclonal (nuclear, in green) and the Anti-SGO2 polyclonal (nuclear, in magenta) antibodies.
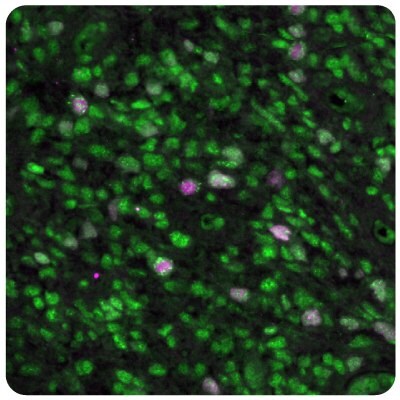
Figure 9B.Multiplexed IHC-IF staining of human glioblastoma samples using the Anti-EZH2 monoclonal (nuclear, in green) and the Anti-SGO2 polyclonal (nuclear, in magenta) antibodies.
Table 3 shows a comparison of monoclonal antibodies that target glioma TME markers.
Reliable Antibodies for Targeting Glioma Markers
Prestige Antibodies® powered by the Human Protein Atlas project provides over 21,000 primary antibodies targeting the majority of human proteins. All our products are rigorously evaluated for specificity, reproducibility, and performance and characterized in multiple applications.
Prestige Antibodies® polyclonals are rabbit polyclonal primary antibodies developed within the Human Protein Atlas project. IHC characterization data from 44 normal and 20 cancer tissues is available on the Human Protein Atlas portal. They are available in 25 µL and 100 µL unit sizes.
Prestige Antibodies® monoclonals are mouse monoclonal primary antibodies developed against many carefully selected targets. Clones are selected to recognize only unique non-overlapping epitopes and isotypes. They are also available in 25 µL and 100 µL unit sizes.
References
続きを確認するには、ログインするか、新規登録が必要です。
アカウントをお持ちではありませんか?