Determination of Baicalin, Chlorogenic acid and Forsythin in Shuanghuanglian Oral Liquid acc. to Chinese Pharmacopeia
Dean Duan, Merck R&D
APAC Lab Shanghai, China
Abstract
In this application, Shuanghuang Lian oral liquid, a well-known traditional Chinese medicine (TCM), was prepared following Chinese Pharmacopoeia 2020 and analyzed on a Discovery® HS C18 HPLC column for the content of three key compounds, baicalin, chlorogenic acid, and forsythin (phillyrin). For baicalin the linear range was 0.5 – 200 µg/mL. LOD and LOQ of baicalin is 0.07 µg/mL and 0.23 µg/mL separately. For chlorogenic acid the linear range is 0.1 – 50 µg/mL. LOD and LOQ of baicalin was 0.11 µg/mL and 0.33 µg/mL separately. For forsythin the linear range was 0.2 – 60 µg/mL. LOD and LOQ of baicalin was 0.02 µg/mL and 0.07 µg/mL separately. The SPE cleanup for the forsythin sample showed good recoveries of 88%. The method met the requirements of the Chinese Pharmacopeia 2020. The Discovery® HS C18 HPLC column can be used to determine the baicalin, chlorogenic acid, and forsythin content of Shuanghuang Lian oral liquid.
Section Overview
Introduction
Shuang-Huang Lian oral liquid is a Chinese patent medicine. It is composed of honeysuckle, scutellaria, and forsythia. The determination of baicalin, chlorogenic acid, and forsythin (phillyrin) as key compounds in the oral liquid by HPLC is listed in the Chinese Pharmacopeia 2020.1 Here we describe three dedicated analytical methods using a Discovery® HS C18 HPLC column according to the pharmacopeia monograph.

Experimental
HPLC conditions and sample preparation applied for the three analytes of interest are shown in Tables 1–3. For baicalin and chlorogenic acid, a dilution and filtration were applied, for forsythin, a clean-up by SPE (interference removal, chemical filtration) using a neutral alumina as adsorbent was used.
Sample Preparation by SPE for Forsythin Determination in Oral Liquid
For the determination of forsythin, an additional cleanup by SPE was applied.
Standards Preparation for Calibration
The calibration standards were prepared according to these procedures:
Diluent: Add 50 mL of methanol and 50 mL of water into a measuring cylinder. Mix well to prepare 50% aqueous methanol solution as diluent.
For Baicalin:
- Weigh ~10 mg of baicalin reference material into a 10 mL volumetric flask.
- Add ~8 mL of diluent and sonicate for 5 mins.
- Top-up to mark with diluent and mix well to prepare 1 mg/mL of baicalin stock solution.
- Dilute to 0.1, 0.2, 0.5, 1. 2, 5, 10, 50, 100 and 200 µg/mL with diluent.
For Chlorogenic acid:
- Weigh ~10 mg of chlorogenic acid reference material into a 10 mL volumetric flask.
- Add ~8 mL of diluent and sonicate for 5 mins.
- Top-up to mark with diluent and mix well to prepare 1 mg/mL of chlorogenic acid stock solution.
- Dilute to 0.1, 0.2, 0.5, 1. 2, 5, 10, 20, 40 and 50 µg/mL with diluent.
For Forsythin:
- Weigh ~10 mg of forsythin reference material into a 10 mL volumetric flask.
- Add ~8 mL of diluent and sonicate for 5 mins.
- Top-up to mark with diluent and mix well to prepare 1 mg/mL of forsythin stock solution.
- Dilute to 0.2, 0.5, 1. 2, 5, 10, 30, 40 and 60 µg/mL with diluent.
Method Suitability / System Suitability Criteria
Acceptance Criteria for Standard Solutions:
- Theoretical plate number calculated for baicalin peak: NLT 1500
- Theoretical plate number calculated for chlorogenic acid peak: NLT 6000
- Theoretical plate number calculated for forsythin peak: NLT 6000
Results & Discussion
The chromatographic results for the three compounds under investigation are displayed in Figures 1–5. The chromatographic data of the separately injected standard solutions is summarized in Table 5.
As an example of the calibration curves, the one for baicalin is displayed in Figure 6.
The methods displayed good reproducibility with RSDs of 0.98%, 0.27%, and 0.98% (Table 6), as well as good linearity and sensitivity (Table 7). In summary, the results for the three analytes determined are:
Baicalin:
- The theoretical plates is 10377 (>1500). It meets the requirements of the Chinese Pharmacopeia 2020.
- The Limit of Detection (LOD) for baicalin in Shuanghuang Lian oral liquid is 3.72 µg/mL, and the Limit of Quantification (LOQ) is 11.27 µg/mL. The range from 0.5 to 200 µg/mL is linear with an R2 of 0.9999.
Chlorogenic acid:
- The theoretical plates is 13128 (>6000). It meets the requirements of the Chinese Pharmacopeia 2020.
- The LOD for chlorogenic acid in Shuanghuang Lian oral liquid is 2.72 µg/mL, and the LOQ is 8.24 µg/mL. The range from 0.1 to 50 µg/mL is linear, with the R2 being 0.9982.
Forsythin:
- The theoretical plates is 8258 (>6000). It meets the requirements of the Chinese Pharmacopeia 2020.
- The LOD for forsythin in Shuanghuang Lian oral liquid is 0.11 µg/mL, and the LOQ is 0.33 µg/mL. The range from 0.2 to 60 µg/mL is linear, with the R2 being 0.9939.
- The average SPE recovery (n = 3) for forsythin was assessed using an oral liquid sample spiked at 75 µg/mL and was determined to be 88% (Table 8).
- The used oral liquid showed a forsythin content below LOD (Figure 4). The chromatogram for a sample spiked at 75 µg/mL is shown in Figure 5.
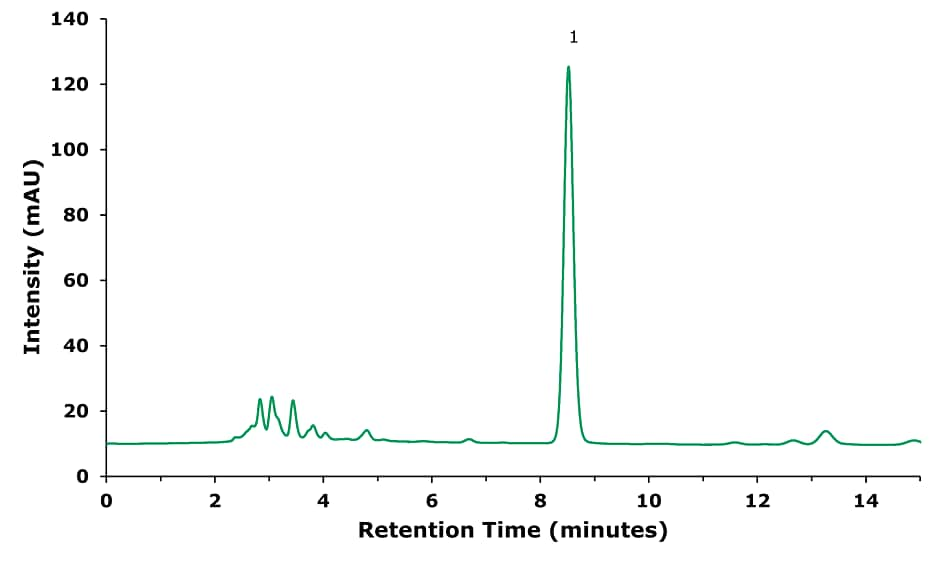
Figure 1.Sample Solution- Sample treated following procedure in baicalin (1) sample preparation part (Table 1).

Figure 2.Sample Solution- Sample treated following procedure in chlorogenic acid (1) sample preparation part (Table 2).

Figure 3.Chromatogram of forsythin (1) standard solution 30 µg/mL.

Figure 4.Sample Solution - Sample treated following the procedure in forsythin sample preparation part (Table 3). The used oral liquid showed forsythin content below LOD.

Figure 5.Oral liquid sample spiked with forsythin (1) at 75 µg/mL - Sample treated following the procedure in forsythin sample preparation part (Table 3).
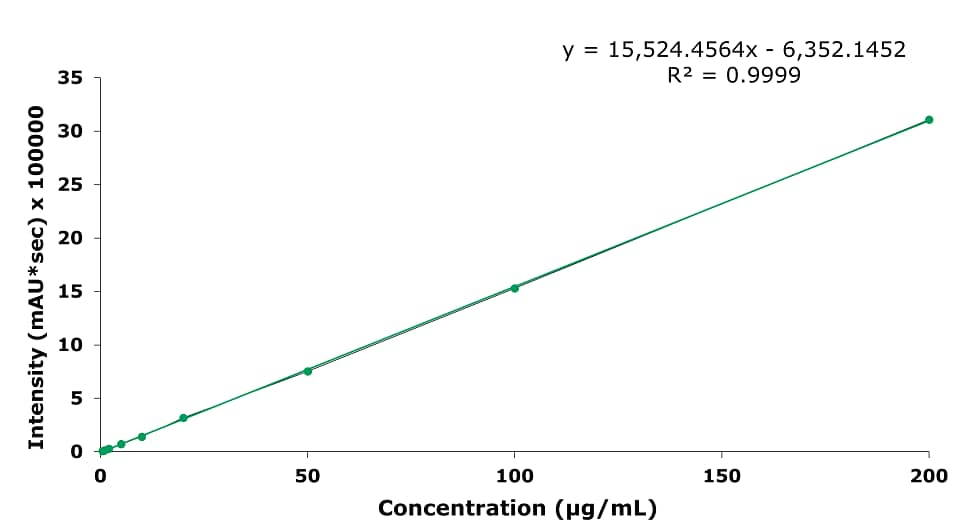
Figure 6.Calibration curve for baicalin.
Conclusion
The three HPLC methods using the Discovery® HS C18 column met the system suitability criteria listed in the Chinese Pharmacopeia monograph. The column is therefore suitable for the determination of baicalin, chlorogenic acid and forsythin in Shuanghuang Lian oral liquid. For the determination of forsythin, the used Supelclean™ LC-Alumina-N SPE Tube for clean-up of the oral liquid sample provided an average recovery (n=3) of 88%, which is suitable for the method.
See more applications on Pharmaceutical Analysis & Quality Control.
References
続きを確認するには、ログインするか、新規登録が必要です。
アカウントをお持ちではありませんか?