Screen Cell-Based Immunotherapeutics for Microbial Contaminants Using the Milliflex® Rapid System
Processing of mammalian cells with lysis buffer to enable filtration and microbial detection
Immunotherapy has become an established treatment for many forms of cancer. Some cancer treatments involve the administration of activated T cells. After production, these immune cells should be released for use and administered to patients as fast as possible to ensure optimal efficacy. However, it is also essential to ensure the therapeutic’s microbiological safety.
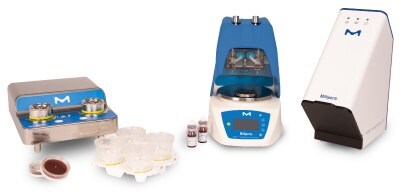
Milliflex® Rapid System 2.0: Automated system for the rapid and accurate detection of microbial contaminants.
MILLIFLEX® RAPID SYSTEM
The Milliflex® Rapid System is an automated ATP-based solution for the rapid detection, imaging, and quantification of viable microbial contaminants in filterable samples throughout the manufacturing process. The system's results help to improve process control, product yield, and the timely release of final products.
RAPID MICROBIAL TESTING FOR SHORT-SHELF-LIFE PRODUCTS
Chapter 10 Quality Control of the EU’s recently revised GMP Annex 1 Manufacture of Sterile Medicinal Products states that, when manufacturing short-shelf-life products, rapid/alternative methods should be considered for the detection of contaminant microbes.
The amounts of sample USP <71> stipulates for release testing of classical pharmaceutical products cannot be applied to many short shelf-life products because batch sizes are often too small to meet these quantitative requirements. Therefore, the EP’s chapter 2.6.27 Microbial Examination of Cell-based Preparations lists sample quantities and gives examples of the release test’s sample size for cell preparations. This is why the required sample size should always be considered when selecting release test technology.
USP published the standard <1071> Rapid Microbial Tests for the release of sterile short shelf-life products, which explains the requirements and the equipment to be used for rapid technologies in the release testing of short shelf-life products. It explicitly discusses the use of ATP (adenosine triphosphate) bioluminescence technologies. The section Adenosine Triphosphate Bioluminescence states “For a rapid microbial test for the release of sterile short-life products, an enrichment culture either in liquid media to reach a threshold ATP level or on a membrane filter on solid media for the formation of microbial colonies could be used with an incubation time of 2–7 days”.
MILLIFLEX® RAPID SYSTEM FOR MICROBIAL TESTING
In this study, two different immune cell preparations (designated cell types A, B) were evaluated for their compatibility with the Milliflex® Rapid System to establish a method for rapid sterility testing in accordance with the applicable current chapters of the European Pharmacopoeia (EP) and United States Pharmacopeia (USP).
The study evaluated the performance of the Milliflex® Rapid System to detect microbial contamination. It investigated bioluminescent background, potential inhibitory effects, microbiological detection and recovery, and filterability.
MILLIFLEX® RAPID SYSTEM TESTING WORKFLOW
The Milliflex® Rapid workflow includes an immunotherapeutic cell lysis step to enhance sample filterability, followed by sample filtration, transfer of the filtration membrane onto an RSTM media plate, and incubation for growth.
After incubation, the membrane filters are separated from the media, air-dried, and placed into the Milliflex® Rapid AutoSpray Station for reagent application. The Milliflex® Rapid System’s Detection Tower and its software automatically detect and count the microcolonies.
Sample Preparation and Cell Lysis
As each cell-based sample has specific properties, it is crucial to determine a suitable sample preparation SOP ensuring optimal filtration and thus reliable test results. This was successfully done for each of the two cell preparations by applying a specific cell lysis protocol.
Mammalian cell culture samples present a unique detection challenge as the Milliflex® Rapid ATP bioluminescence-based technology is not selective for microbial ATP. Mammalian ATP released from the cells captured on the membrane produces an interfering signal resulting in false-positive counts. To enable the exclusive detection of microbial ATP and achieve accurate counting of CFUs, it is essential to eliminate this mammalian ATP background. A method was developed to selectively lyse mammalian cells and remove the ATP they have produced prior to the step of detecting microbial ATP. Samples were pretreated with a selective mammalian cell lysis solution and with the enzyme apyrase.
To demonstrate the selectivity of the MCLB (Mammalian Cell Lysis Buffer) toward mammalian cells only, samples were spiked before the cell lysis process started, each with an inoculum of <55 CFUs.
Each sample was incubated in a sterile cell lysis solution containing apyrase that makes the sample filterable by lysing the mammalian cells and removing free ATP while any contained microorganisms stayed intact.
Filtration and Membrane Transfer
After incubation for cell lysis, all samples (three samples per cell type preparation and per microorganism strain: one for traditional method reading, two for Milliflex® Rapid reading) were filtered through a Milliflex Oasis® Rapid filtration device with a PVDF 0.45 μm membrane to capture the microorganisms present in the sample. Subsequently, the membrane filters were each transferred onto an RSTM media cassette and incubated for growth.
Counting the Microcolonies
Bioluminescence recovery defines the microcolonies that the rapid test method detects as a percentage of those that the traditional method detects (equivalency criterion: 70% to 130%). In the traditional Milliflex® method, incubation of the Milliflex® Rapid membrane on a medium continues until the colonies are visible and countable with the naked eye.
RESULTS OF MICROBIAL TESTING USING THE RAPID DETECTION SYSTEM
- Suitable cell lysis and filtration procedures were developed for both cell types. The bioluminescence background analysis was performed to avoid false-positive results. It verified that non-microbial sample constituents and filtration liquids did not cause bioluminescence.
- The spiking procedures demonstrated the absence of antimicrobial effects of the reagents or cell preparations. Any organisms present in the sample would be adequately detected during a routine sterility test.
- Also, no substances inhibited the detection and quantification of the microorganisms by bioluminescence. The chosen bacteria, yeast, and mold strains all showed recovery rates above the 70% minimum criterion and were all detectable after 1 to 3 days of incubation.
ROBUST COMPATIBILITY AND DATA CONSISTENCY WITH THE MILLIFLEX® RAPID SYSTEM
Two different short shelf-life products, containing cells type A and B, underwent compatibility testing with the Milliflex® Rapid microbial testing method. Both were found to be fully compatible with this rapid detection and enumeration technology.
The study demonstrated the filterability of the two cell-type preparations using Milliflex® Rapid consumables after performing a cell lysis step. For each cell type, a testing procedure was developed, and a bioluminescence background analysis was performed. All analyses showed the absence of any background interference with microbial detection and enumeration. It was also shown that neither the sample nor any reagents exert antimicrobial activity that would lead to false-negative results. This is due to the rinsing scheme of the rapid method.
No inhibition was discovered in the rapid method’s ability to detect and quantify microcolonies by bioluminescence. The selected bacteria, yeast, and mold strains all exhibited recovery rates above the 70% minimum criterion (Table 1). It can thus be concluded that the cell lysis step, performed to prepare for filtration did not falsify the detection results.
The data presented here were obtained using the original Milliflex® Rapid System, which has since been replaced by a state-of-the-art Milliflex® Rapid System 2.0. The new version includes a new detection tower and software. However, the four essential steps of the workflow, which might have had the potential to impact detection results, remain fundamentally the same.
A COMPLETE SERVICE OFFERING
Take advantage of our decades of expertise in both technology and services:
- Method Development Services: Optimize or simplify your method for an efficient validation and cost-effective testing. Validation Protocols and On-Site Validation Services: Get support from our experts to have your new QC system quickly and efficiently validated. Digital validation protocols are also available via our Val@M™ application.
- Service Plans: Ensure optimum performance and reliability of your system thanks to our Maintenance and Repair Services, performed at your site or at our repair center.
- Training Services: Benefit from our training packages including in-depth review of regulatory requirements, their validation and implementation.
続きを確認するには、ログインするか、新規登録が必要です。
アカウントをお持ちではありませんか?