表1PEGマクロモノマーのさまざまな末端基
組織工学や薬物送達などをはじめとするバイオテクノロジー分野の進歩に伴い、さまざまな機能性生体材料の需要が増大しています。過去数十年にわたり、高分子生体材料の研究では、他の用途のために開発されたポリマーの生体適合性の確認やその加工技術の開発(エレクトロスピニング、溶媒キャスト/孔物質溶出(porogen leaching)法、3次元印刷など)に重点が置かれてきました。最近では生体医学用に特化した材料の合成、すなわち合成タンパク質や糖類似体、水媒体との相溶性の高いポリマーの合成に加え、自然界に存在するポリマーの化学修飾(ゲル化やin vivoでの安定性の強化など)へと研究の流れが変化しています。この十年の間では、細胞足場としての利用や治療薬の送達を目的として設計された生体材料の開発などが行われています。
研究者から強い関心が寄せられている生体材料の1つに、ヒドロゲルがあります1。ヒドロゲルは2次元および3次元の細胞足場として広く研究されていますが、それは化学的にも物理的にも本来の細胞の環境に非常に近い状態を模倣しているためです2。ヒドロゲルは、合成ポリマー(ポリエチレングリコール、ポリヒドロキシエチルメタクリラートなど)や天然に存在するポリマー(コラーゲン、ヒアルロン酸、ヘパリンなど)から合成され1b、その高い水分含有量と、細胞、タンパク質、DNAの存在下で作製可能であるために細胞培養の3次元モデルとして有用な材料です。構成する材料の反応性にもよりますが、pH3、温度4、クーロン相互作用、共有結合/非共有結合性相互作用5、または重合反応を用いることで、ゲル化を行うことが可能です。
ポリエチレングリコール(PEG)は親水性ポリマーのひとつで、架橋してネットワークを構成させることで大量の水を保持することができます。一般的にPEGは免疫反応を引き起こさないため、生物学的応用に適した材料です6。1970年代以降、治療用タンパク質やペプチドを修飾することによる溶解度の向上や毒性の低下、循環滞留性の向上のためにPEGは使われてきました7。そして、1970年代後半になると、PEGヒドロゲルを用いた細胞培養の実験が始まりました。PEGヒドロゲルは化学組成が明確であり、その合成や化学修飾に多様な化学を利用することができます。
PEGはエチレンオキシドのリビングアニオン開環重合によって合成されます。そのため、多様な末端基(例えば、アルコール、メチルエーテル、アミン、N-ヒドロキシスクシンイミジル(NHS)エステル)を持ち、広い範囲にわたってさまざまな分子量を持つ、よく定義された(多分散性が低い)PEGを容易に得ることができます。
ヒドロゲルの形成にはPEGの架橋が必要です。初期の頃は、電離放射線を用いて非特異的に架橋されていました8。現在では、PEGヒドロゲルの合成には、PEGマクロモノマーの反応性鎖末端を利用して共有結合的に架橋させる方法が一般的に用いられています。
アクリラート、メタクリラート、アリルエーテル、マレイミド、ビニルスルホン、NHSエステル、ビニルエーテル基などの反応性鎖末端を持つPEGマクロモノマー(表1)は、入手の容易な出発物質から簡便に合成されます。PEGのアルコール鎖末端は、塩基存在下で、塩化アクリロイル、塩化メタクリロイルなどの酸性塩化物を用いてエステル化することができます。PEG鎖末端は、塩基性条件下で、2-chloroethyl vinyl etherやallyl bromideなどのハロゲン化アルキルと反応させることでエステル化が可能です。PEGジビニルスルホンは、PEGを大過剰のジビニルスルホンとカップリングさせるか、あるいは、クロロエチルスルホン鎖末端を調製した後に、塩基を除くことでジビニルスルホン基を導入する多段階プロセスによって合成します9。

Chart 1.End groups of different PEG macromers.
Macromers can be homobifunctional or heterobifunctional.Homobifunctional macromers are typically used to form networks, while heterobifunctional macromers may be used to tether a therapeutic molecule into a hydrogel network.
The cross-linking mechanism to form hydrogels depends on the identity of the chain ends of PEG macromers.In most cases, cross-linking occurs when the reactive vinyl chain ends are polymerized, usually with a free radical initiator.For example, polymerization of macromers can be initiated using redox-generated radicals (e.g., ammonium persulfate and TEMED), or radicals generated with light (e.g., Irgacure® 651, λ=365 nM Scheme 1).Acrylate and methacrylate chain ends undergo chain polymerization.In step growth network formation, a multifunctional (f>2) cross-linker reacts with the PEG chain ends in a stoichiometric manner; alternatively, multifunctional PEGs (f>2) can be crosslinked with difunctional crosslinkers (Scheme 1).Acrylate, methacrylate, vinyl sulfone, maleimide, vinyl ether and allyl ether are all capable of step growth network formation, through conversion to thiols depending on reaction conditions.Typical cross-linkers may include thiol or amine moieties.Mixed-mode polymerizations are the result of both mechanisms occurring in the same reaction vessel; acrylate and methacrylate groups can undergo mixed mode network formation.Both mechanisms of hydrogel formation can be used to encapsulate live cells, and both mechanisms allow for the reactive incorporation of peptides, proteins and other therapeutics.
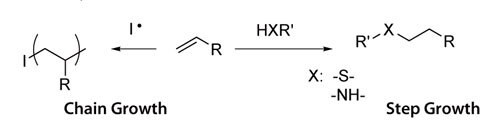
Scheme 1.Chain growth and step growth reactions.
The mesh structure that results from different mechanisms is depicted in Figure 1.In chain growth networks, a kinetic chain is formed at the crosslink site, while in step growth networks, the crosslink sites bear the same functionality as the multifunctional cross-linker, neglecting defects.In both chain and step growth, network defects such as loops, permanent entanglements and dangling chain ends may exist.The chemical identity of the macromer and the mechanism of hydrogel formation are both important as each influences the cross-link density of the hydrogel network.Material properties that are important to 2D and 3D culture are easily controlled through the chemistry of hydrogel formation.As cross-linking density increases, mesh size decreases, swelling ratio decreases, and storage modulus increases.Varying the molecular weight of the PEG macromer results in coarse control over hydrogel properties (large differences in cross-linking density).Varying the reaction mechanism used to produce the hydrogels results in fine control over hydrogel properties (can be used to tune cross-linking density of a system).

Figure 1.Formation mechanism affects hydrogel network structure and network defects.
In order to use 3D hydrogel scaffolds to study cell differentiation and tissue evolution, it is critical to be able to control the physical and chemical properties of the gel in a spatially and temporally controlled manner.10 Polymeric material properties are typically changed through polymerization/cross-linking (bond forming events) or through controlled degradation and/or release (bond breaking events).Bond forming events typically often use small molecule reagents (initiators, catalysts, monomers, ligands to be conjugated to the material) while bond breaking typically does not rely on exogenous reagents.Small molecules often have more adverse effects in vitro and in vivo than polymeric reagents so many research groups use degradation as a tool for in situ manipulation of polymeric biomaterials.
The mechanism of degradation most commonly utilized in hydrogels is hydrolysis, in which a molecule of water adds to the polymer backbone, causing chain scission.Anhydrides, esters and amides are all susceptible to hydrolysis.Anyhydrides typically hydrolyze too quickly, and the uncatalyzed hydrolysis of amides is too slow, so most hydrogels that degrade hydrolytically utilize ester linkages.In order to obtain hydrolytically degradable hydrogels with physiologically relevant time scales of degradation, researchers typically functionalize PEG with degradable ester linkages using lactide or glycolide segments.
Alcohol chain ends on PEG can initiate ring-opening reactions of 3,6-dimethyl-1,4-dioxane-2,5-dione and 1,4-Dioxane-2,5-dione to generate PEG-lactide and PEG-glycolide, respectively (Scheme 2).11 The ring-opening reaction is typically catalyzed by tin(II)-2-ethylhexanoate,12 although the reaction is also easily accomplished using dimethylaminopyridine as a catalyst,13 which may be easier to remove than the residual tin.The alcohol chain ends of PEG-lactide or PEG-glycolide are easily functionalized with reactive double bonds such as acrylate and methacrylate.

Scheme 2.Synthesis of PEG-lactide and PEG-glycolide.
Although ester linkages are enzymatically degradable, most researchers utilize sequence-specific enzymatic degradation of peptides incorporated into hydrogels rather than non-specific enzymatic degradation of esters and amides.Hubbell′s group pioneered this approach16 by incorporating matrix metalloproteinase (MMP) sensitive linkages into hydrogels via Michael addition of cysteine-functionalized peptides across acrylates, maleimides and vinyl sulfones (Scheme 3).17
MMP-degradable linkages have also been used to tether therapeutic agents into hydrogels.For example, growth factors such as vascular endothelial growth factor (VEG-F) can be released via enzymatic degradation of an MMP-sensitive tether to induce angiogenesis.18
In both hydrolysis and enzymolysis, the rate of degradation is predetermined by the chemistry of the macromer.In hydrolysis, the degradation rate of the material is pre-engineered through the identity (e.g., hydrophobicity or hydrophilicity) and number of the hydrolysable groups, and cannot be changed once the material is fabricated.In enzymolysis, the degradation typically occurs in an area local to the cells producing the enzyme.While hydrolysis and enzymolysis are both effective methods for sustained hydrogel degradation and sustained release of therapeutic agents, the rate of release cannot be adjusted or arrested after the hydrogel is fabricated, and release is not spatially controlled.

Scheme 3.Enzymatically degradable hydrogels via Michael addition of cysteinecontaining peptides to vinyl sulfone groups.
In contrast to hydrolytically and enzymatically degradable linkages, photodegradable linkages allow precise spatial and temporal control over degradation and release.While many researchers have reported photopolymerizable hydrogels, and photofunctionalizable hydrogels, very few reports exist of biocompatible photodegradable hydrogels.Kloxin and Kasko reported photodegradable hydrogel networks formed from 2-methoxy-5-nitro-4-(1-hydroxyethyl) phenoxybutanoate-containing PEG macromers (Scheme 4)19; the photodegradation behavior of the ortho-nitrobenzyl (o-NB) linker group is well-characterized.Hydrogels formed from the photodegradable macromer show bulk degradation upon exposure to light that is dependent on exposure time, wavelength, and light intensity.When the light is shuttered, degradation is arrested; the sample continues photolyzing once light exposure resumes. hMSCs (human mesenchymal stem cells) encapsulated in a hydrogel containing the photo-releasable cell-adhesive ligand RGDS (Arg-Gly-Asp-Ser) differentiate down the chondrogenic pathway when the RGD is released at day ten (corresponding to the downregulation of fibronectin during chondrogenesis).Surface erosion and through-gel lithography of this degradable hydrogel can be used to form features over a range of lengths scales, from 10-7 m to 10-2 m or larger.20 Partial degradation in a local area results in decreased cross-link density and increased swelling, providing a means to etch softer features onto a hydrogel that protrude out from the gel.

Scheme 4.Photodegradable o-NB moieties incorporated into hydrogel backbone and for therapeutic agent release.
In addition to single photon photolysis, the o-NB containing hydrogels are also susceptible to two-photon photolysis, allowing for 3D etching.19-20 In single photon reactions, any area exposed to the light will react.In contrast, multi-photon lithography should occur only where multiple photons are simultaneously absorbed, which occurs at the focal volume of the light source (inset).Typical wavelengths in single photon lithography of biomaterials range from long wave UV (≥365 nM) into the visible region, while two-photon lithography uses IR light (typically ~740–800 nM).IR light is more biocompatible and less destructive to live tissues and offers greater penetration depth.The probability of twophoton absorption occurring is also tightly limited to the focal point of the focused light, rather than along the entire path of the light, providing 3D control over excitation.Both single- and multi-photon reactions have the potential to pattern materials with features smaller than 500 nM, much smaller than the size of a mammalian cell.21 This represents an unprecedented level of spatial control over hydrogel scaffold structure and chemistry.

Figure 2.Single photon photolysis (left) occurs in the entire area of the hydrogel exposed to UV-visible light, and two photon photolysis (right) results only in the area where simultaneous absorption of two photons of IR light occurs.
The o-NB linker can also be used to tether therapeutic agents into hydrogels for delivery to live cells.Griffin et al. demonstrated the controlled release of fluorescein tethered into a hydrogel through an o-NB-PEG macromer.22 The release of this model therapeutic as a function of light exposure at multiple wavelengths (365–436 nM), intensities (5–20 mW/cm2) and durations (0–20 minutes) was quantified.While the fastest release occurs at 365 nM (which corresponds to a higher molar absorptivity of the o-NB linker at that wavelength), significant release is also seen at 405 nM; the release is easily modeled from physical constants of the molecules (such as molar absorptivity).Light attenuation allows the facile formation of chemical and mechanical gradients in these systems.
Poly(ethylene glycol) is a readily available, easily modifiable polymer.It has found widespread use in hydrogel fabrication, including as 2D and 3D scaffolds for tissue culture.Degradable linkages are easily introduced into PEG hydrogels.Hydrolytically degradable gels allow for sustained material degradation and/or therapeutic agent release.Degradation and release is cell-dictated in enzymatically degradable gels.Photodegradation allows for real-time user tailored external manipulation of the chemical and physical properties of hydrogels.
続きを確認するには、ログインするか、新規登録が必要です。
アカウントをお持ちではありませんか?