ATRP Ligands & Initiators: Clean Functional Polymers
Wojciech Jakubowski, Nicolay V. Tsarevsky, Patrick McCarthy
Krzysztof Matyjaszewski ATRP Solutions, Inc. 166N. Dithridge Street, Suite G4, Pittsburgh, PA 15213
Material Matters 2010, 5.1, 16.
Introduction
Atom transfer radical polymerization (ATRP)1-4 has emerged as one of the most successful synthetic techniques for the preparation of polymers with predetermined molecular weights, narrow molecular weight distributions, and high degrees of chain end functionalities (Scheme 1). The unprecedented control over molecular architecture afforded by the ATRP enables preparation of systematic polymer libraries.5 Scheme 1 exemplifies a systematic library of star-shaped polymers, where the polymers in each row have the same arm size and those in each column have the same number of arms. Such libraries can provide important data for understanding the relationships between polymer structure and physical properties or function.

Scheme 1.Schematic illustration of the ATRP (top) and an example of a star-shaped polymer library.
Catalysts for ATRP
ATRP is catalytic process using a metal complex, in which the transition metal Mt can exist in two different oxidation states. The lower oxidation state metal complex Mtz/L (L is a ligand) reacts with the ATRP initiator (alkyl halide RX) to yield a radical R• and the corresponding higher oxidation state metal complex with a coordinated halide anion X-Mtz+1/L, in a process termed activation, proceeding with the rate constant, kact. The latter complex can transfer the halogen atom back to the radical, re-generating the alkyl halide and the lower oxidation state metal complex. The radicals can react with the monomer M (generating polymer with the rate constant of propagation kp), with each other (termination with the rate constant, kt) or with X-Mtz+1/L (deactivation with the rate constant, kdeact). The last step, which distinguishes ATRP from conventional radical polymerization, yields the halogen-terminated polymeric dormant state, which can be reactivated in a reaction with Mtz/L. If the deactivation process is efficient (i.e., high value of kdeact) and if all polymer chains are initiated within a short period by appropriate selection of the alkyl halide initiator, the resulting polymer will be characterized by a narrow molecular weight distribution. Additionally, it is desirable to use an active catalyst with a high value of the ratio of kact / kdeact, termed the ATRP equilibrium constant, kATRP, to ensure fast polymerization. The rate constants kact and kdeact depend on both the transition metal and the ligand. Rules for the rational selection of active catalysts for ATRP for various reaction media and monomers have been developed.2,6
Various metals and ligands have been successfully employed as catalysts in ATRP, but the most often used are the catalysts based on copper (the two oxidation states are CuI and CuII) and N-containing ligands. One drawback of the classical ATRP is the use of high amounts of the catalyst.4 The obtained polymers are well-defined in terms of molecular weight distribution and chain-end functionality but require tedious purification to remove the catalyst. Although various methods for catalyst removal have been developed,2,7 the extra purification step is associated with longer time needed to obtain the final product, and with generation of waste, both of which increased the cost of the materials prepared by ATRP. However, the use of ligands such as tris[2- (dimethylamino)ethyl]amine (Me6TREN, Prod. No. 723142) and tris(2-pyridylmethyl)amine (TPMA, Prod. No. 723134) alleviates this problem (Figure 1). These ligands can be used in new techniques called Activators ReGenerated by Electron Transfer (ARGET)8,9 and Initiators for Continuous Activator Regeneration (ICAR)10 which allow to decrease amount of catalyst to only few, often single-digit, ppm. For comparison, 1,000 to 10,000 ppm were used in traditional ATRP. For many applications, in these new systems the residual copper can be left in the final colorless products. Both techniques employ a reducing agent: a radical initiator such as AIBN in ICAR ATRP;10 and tin(II) ethylhexanoate8,9,11-13 (Prod. No. S3252), ascorbic acid,14glucose,9 hydrazine,10 or Cu(0)15 in ARGET ATRP. These reducing agents allow for regeneration of the lower oxidation state metal complex, which would normally be irreversibly converted to the higher oxidation state complex due to radical termination by a process dubbed "persistent radical effect".16

Figure 1.Ligands for Cu-mediated ATRP using ppm amounts of catalyst. 723142; 723134
The ARGET and ICAR ATRP processes allow chemists to reduce the amount of catalyst more than one thousand times and the polymers obtained are white or colorless. These processes also allow preparation of well-defined block copolymers,12 polymers with high molecular weight,11,17 high chain-end functionality11 and adjustable molecular weight distribution.18 In addition, since the level of control in an ARGET and ICAR ATRP is only weakly affected by an excess of reducing agent, the reaction can be successfully carried out in the presence of limited amounts of air.13 Reactions can be carried out without deoxygenation, in flasks fitted with rubber septa or even in simple jars. This was demonstrated in our laboratory by placing functionalized wafers in one of these vessels and growing very dense polymer brushes (~0.4 chain/nm2), including block copolymer brushes, without any deoxygenation. ATRP stops after opening the vessel to air but starts again when a sufficient amount of reducing agent is added to the closed flask. This polymerization process does not require any special skills and is especially well-suited for grafting from larger surfaces, but can also be applied for preparation of any other polymer materials. Only very active catalysts derived from Me6TREN and TPMA can be used in these new techniques. Figure 2 presents the kinetic plot, evolution of molecular weights and polydispersities with conversion and GPC traces for polymerization of styrene (St) with 50 ppm of CuIIBr2/TPMA catalyst in the presence of AIBN as reducing agent. Molecular weight control is excellent and follows theoretical values based on quantitative initiation. The polymer, after precipitation in hexane, appeared as a white solid powder containing only 5 ppm of the residual catalyst. If more Cu removal is needed, the ATRP pure® resin can be used.5,19

Figure 2.ICAR ATRP of styrene (St) using 50 ppm of catalyst. (a) Kinetic plot (b) Molecular weight and polydispersity as a function of conversion (c) Evolution of GPC traces (d) Photograph of polymer after precipitation in hexane.
Typical ICAR ATRP procedure
The following is a procedure employing very low concentration of copper catalyst that yields well-defined polystyrene macroinitiator (PSt-Br) with degree of polymerization 100. As shown in Figure 2, the polymerization is well-controlled: the linear first order kinetic plot of monomer consumption indicates constant number of active species and the increase of molecular weights with conversion is characteristic of a living process. Moreover, the obtained polymer was virtually colorless without the use of any special purification methods other than simple precipitation in hexane.
- Add CuBr2 (7.8 mg, 3.5 × 10-2 mmol) and TPMA (10.1 mg, 3.49 × 10-2 mmol) to a 10 mL flask equipped with magnetic stirring bar.
- Add DMF (4 mL) to solubilize CuBr2/TPMA. Stir for 10 min to obtain a homogeneous yellowish solution.
- Add St (80.0 mL, 0.698 mmol), AIBN (0.153 g, 0.0931 mmol) and ethyl 2-bromoisobutyrate (0.68 mL, 4.65 mmol) to a 200-mL round bottom flask equipped with a magnetic stirring bar.
- Transfer the catalyst solution to the 200 mL round bottom flask reactor.
- Close the flask reactor with glass adapter (with glass stopcock and a rubber septum). Stir the solution while purging with nitrogen for 1 h.
- Place the flask in an oil bath at 70 oC. To follow the progress of the reaction, samples can be withdrawn with a stainless steel needle. The samples can be analyzed by GC or NMR (monomer conversion) and SEC (molecular weight and polydispersity).
- After 20.5 h*, the monomer conversion reaches 69 %. Mn = 9,700 g/mol, PDI = 1.11. The reactor is opened to air and allowed to cool to room temperature.
- Dilute the polymer with THF (100 mL) and precipitate into 2 L of hexane.
- Dry the produced polymer at 45 oC to constant weight (ca. 24 h).
* Time of the reaction may vary depending on a type of used equipment and purity of chemical reagents.
It is interesting to compare the results of ICAR ATRP employing Me6TREN or TPMA with the process under similar conditions but with other catalysts, derived from ligands, such as the traditionally used derivatives of 2,2-bipyridine (bpy) (ex., Prod. No. 482250) or N,N,N′,N”,N”- pentamethyldiethylenetriamine (PMDETA, Prod. No. 369497). Me6TREN and TPMA were successfully used in ICAR and ARGET ATRP of various monomers such as styrene,9-11 methyl acrylate,15 butyl acrylate,8methyl methacrylate,8,12 butyl methacrylate,20 dimethylaminoethyl methacrylate21 and acrylonitrile.17,22 They can also be used in classical ATRP of coordinating monomers such as, for example, 4-vinylpyridine (Prod. No. V3204). Rate of ICAR ATRP is not affected by the catalysts but it is defined by the rate of decomposition of the radical initiator and can be significantly accelerated at higher temperatures.
Block Copolymers
Block copolymers continue to remain a subject of intense research and technological interest due to their unusual and useful properties.23,24Current and potential high-technology applications of block copolymers are based on their ability to self-assemble, in bulk as well as in selective solvents, into ordered nanostructures. For example, block copolymers with hydrophilic and hydrophobic segments self-assemble, both in the solid state and in the solution, to generate a variety of nano-scale structures. The structures range from simple micellar or lamellar to complex gyroid. Recent studies on block copolymer self assembly demonstrated that nano-scale morphology is highly dependent on block chain length, chain length ratios, polydispersity index, and block composition. Therefore, it is essential to precisely control the degree of polymerization of each segment in the block copolymer and achieve narrow molecular weight distribution. ATRP is a convenient technique for preparation of block copolymers because the growing polymer chain contains a stable halogen terminated ω-end that can act as an initiator for chain extension.
As seen from Table 1, only the polymerizations mediated by the complexes of Me6TREN and TPMA (the first two entries) were wellcontrolled and yielded polymers with narrow molecular weight distribution.10 In all cases, only 50 ppm of Cu was employed.

Table 1.ICAR ATRP of St initiated by ethyl 2-bromoisobutyrate (EBiB) in the presence of various Cu-based catalysts.
Figure 3 presents GPC traces of polystyrene-b-poly(t-butyl acrylate) (PStb- PtBA) as an example of synthesis of a block copolymer library.5 In order to synthesize these copolymers ICAR and ARGET ATRP were used with similar conditions as described above. This library can be then converted to polymeric surfactants polystyrene-b-poly(acrylic acid) (PStb- PAA) by deprotection of t-butyl groups. PSt-b-PAA copolymers may be used as polymeric surfactants in many applications such as particle dispersants (organic, inorganic and metals), nano-device delivery vehicles, blend compatibilizers, coatings, surface modifiers, detergents, and emulsifiers. The broad range of compositions and molecular weights provided by each polymeric library synthesized by ATRP allows rapid screening and identification of the optimal structure for the particular application. Several systematic polymeric libraries are now commercially available.19

Figure 3.GPC traces and properties of PSt-b-PtBA block copolymer library.
Initiators for ATRP
ATRP uses simple initiators, mainly alkyl halides R-X (X = Cl, Br).1,25,26 The number-average molecular weight Mn of polymers prepared by ATRP depends on the initial concentration ratio of monomer (M) to initiator as well as the monomer conversion:
Mn = ([M]0 / [RX]0) × Conv × MW(M)
where [M]0 is the initial monomer concentration, [RX]0 is the initial concentration of alkyl halide, Conv is the monomer conversion, and MW(M) is the molecular weight of the monomer. The alkyl halides used as initiators can contain either one or numerous halogen atoms. Depending on the exact initiator structure and the number of halogen atoms, the architecture of the prepared polymers can be varied from linear (using alkyl halides with a single halogen atom), to star-like or brush-like (multiple halogen atoms in the initiator). Star polymers can be generated using initiators with alkyl halide groups attached to a single core (Figure 4), whereas to obtain brush polymers, the halide groups should be attached along the backbone of a polymer or a large molecule or nanoparticle with a high aspect ratio (e.g., a carbon nanotube).

Figure 4.Examples of ATRP initiators yielding polymeric stars. 723177; 723185; 723193; 723207.
Four major strategies exist for the synthesis of polymers with functional groups via ATRP: i) direct polymerization of functional monomers, ii) polymerization of "protected" monomers followed by post-polymerization chemical transformations, iii) the use of functional initiators, and iv) substitutions of the terminal halogen atom. The first two approaches yield polymers with multiple functionalities along the backbone whereas the last two yield end-functionalized polymers. Figure 5 illustrates structures of alkyl halide functional initiators that yield endfunctionalized polymers. Groups such as hydroxy are suitable for the synthesis of polymers that can react with molecules, or surfaces with carboxylic acid groups. Allyl group-containing initiators yield polymers that can participate in hydrosilylation or ene reactions with polymers or surfaces containing Si-H or S-H bonds, respectively. Trichlorosilyl groups react with surfaces containing hydroxy or amine groups (including Si-OH bonds), such as those on the surface of silica particles or glass.Finally, disulfide-containing difunctional initiators yield polymers containing a functional group able to react with gold surfaces, and also gives the polymers the ability to degrade in reducing environments.

Figure 5.Examples of ATRP initiators that can be used to prepare end-functionalized and disulfide containing polymers.723150; 723169; 723215; 723223; 723231.
A much broader variety of functional alkyl halides can be easily custom-synthesized.1 Several concepts for functional polymer architectures that can be prepared using functionalized ATRP initiators are further illustrated in Figure 6. It should be emphasized that the polymers prepared by ATRP contain two chain ends: the α-end (FG) derived from the initiator and the ω-end, which is normally a bromine or chlorine atom. Alkyl halides can participate in a number of nucleophilic substitution reactions, which expands significantly the types of endfunctional polymers accessible through ATRP.25
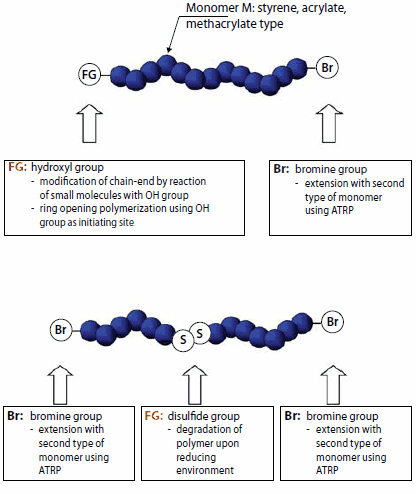
Figure 6.Examples of end-functionalized polymers prepared by ATRP using an initiator with a hydroxy or disulfide functional group (FG).
Summary
The number of materials and commercial products that use polymers either in a pure form or as a part of more complex mixtures, blends, and composites, is countless. The properties and application of polymers depend not only on the molecular size but also on the molecular shape and composition.27 Today, ATRP is one of the most powerful polymer synthetic methods which allows control over molecular architecture, as evidenced by over one hundred patent applications, over a thousand journal articles published annually, and also in a number of commercial products made in US, Japan and Europe. Due to recent advancements in initiation processes (ARGET and ICAR ATRP) it is relatively easy to perform any polymerization reaction and the purification of the final products is now easier, while generating a minimum amount of waste.
References
続きを確認するには、ログインするか、新規登録が必要です。
アカウントをお持ちではありませんか?