Detection of Genomic DNA from Processed Plant Oils and Wheat Flour
E. Sonke, BMSc1, W. Kim, Ph.D1, Y. Haj-Ahmad, Ph.D1,2
1Norgen Biotek Corporation, Thorold, Ontario, Canada, 2Centre for Biotechnology. Brock University, St. Catharines, Ontario, Canada
Product No. E5038
Introduction
Food processing practices are common in today’s agricultural and food industries, and are often harsh on the genetic information of plants and plant products. Commonly produced oils such as olive oil, sesame oil and vegetable oil often contain only trace amounts of genetic information (plant DNA or RNA) following treatment with high pressures and high temperatures, and this DNA is mostly of low quality1. The development of protocols that are able to isolate the trace amounts of high quality DNA from oils is of importance to the olive oil industry and the economies that depend on its production2. Adulteration of pure olive oil with oils of lower cost and value is a growing concern in the industry and these practices can be regulated by establishing and tracing the genetic origin of food products3.
Another growing concern in the minds of food consumers is the introduction of genetically modified organisms (GMOs) into the daily food supply. Vegetable oil producers are currently permitted to incorporate GMOs into their product and although GMOs are not permitted in the wheat industry, GMO wheat strains have recently been detected in North America4,5. Detection of genetic modifications can only be done through DNA analysis, and isolating good quality DNA from processed wheat (wheat flour) can be difficult6.
Norgen Biotek’s Plant/Fungi DNA Isolation Kit has already been proven to isolate high quality DNA from several plant samples, such as tobacco leaves, tomato leaves, grape leaves, peach leaves, plum leaves and pine needles. Furthermore, the DNA isolated using the kit is compatible for use in downstream applications such as qPCR, Southern blotting, SNP analysis and sequencing. This application note illustrates that DNA suitable for downstream PCR analysis can be isolated from olive oil, sesame oil, vegetable oil and wheat flour using Norgen’s Plant/Fungi DNA Isolation Kit. This makes our kit suitable for isolating high quality DNA to be used for testing the genetic authenticity of these food products.
Materials and Methods
DNA Isolation - Oils
Store-bought olive oil, sesame oil and vegetable oil were mixed with an equal volume (500 μL) of Lysis Buffer from Norgen’s Plant/Fungi DNA Isolation Kit and vortexed for 10 minutes at room temperature to allow for adequate mixing. Lysate was then incubated at 65 °C for 10 minutes with intermittent mixing to ensure that aqueous and non-aqueous layers did not separate. To separate the supernatant, lysates were centrifuged at 14,000 rpm for 5 minutes. Approximately 350 μL of supernatant was then mixed with an equal volume of 70% ethanol and this solution was passed through Norgen’s column to bind DNA. Following a series of wash steps DNA was eluted in 50 μL of Elution Buffer and was ready for PCR amplification.
DNA Isolation – Wheat Flour
Store-bought wheat flour was weighed out in 5, 10, 15, 20, 30, 40 and 50 mg aliquots, mixed with 500 µL of Lysis Buffer and 100 µL of Lysis Additive, and vortexed briefly. Lysate was then incubated at 65 °C for 10 minutes to allow lysis. To separate the supernatant, lysates were centrifuged at 14,000 rpm for 5 minutes. Approximately 350 µL of supernatant was then mixed with an equal volume of 70% ethanol and this solution was passed through Norgen’s column to bind DNA. Following a series of wash steps DNA was eluted in 100 µL of Elution Buffer and was ready for PCR amplification.
PCR Amplification
The purified DNA was then used as a template in endpoint PCR reactions. To amplify plant genomic DNA, 2 μL of isolated DNA was added to 18 μL of reaction mixture containing 0.5 μM 18S rDNA primers. Reactions were run using the program; 95 °C for 3 minutes for an initial denaturation, 45 cycles of 95 °C for 15 seconds for denaturation, 60 °C for 30 seconds for annealing and 72 °C for 45 seconds for extension. The reaction was run on an iCycler iQ thermocycler (Bio-Rad).
Gel electrophoresis
For visual analysis of end-point PCR products, the entire volume (20 µL) of the PCR reaction was loaded on to a
1% agarose TAE gel and run for 25 minutes at 150 V alongside Norgen’s HighRanger 1 kb DNA ladder. Gel photos were taken using an AlphaImager™ IS-2200 (Alpha Innotech).
Results and Discussion
Oils
Prior to PCR amplification, the isolated DNA samples were run on 1% agarose TAE gel for visual analysis. The gel showed that very little DNA had been isolated from the various oil samples, and that the DNA had been sheared (data not shown). Whatever quality genomic DNA had been isolated was not detectable by gel electrophoresis.
However, running samples on a 1% agarose TAE gel following end-point PCR indicated that amplification of plant 18S rDNA (520 bp) had occurred in half (1/2) of the replicates for all three types of oil (Figure 1). This indicates that the DNA amount in the processed oil is almost non-detectable, and was present in barely traceable amounts. Similar observations were made by R. Testolin and O. Lain7 (2005), who also indicated that only half of the olive oil DNA samples in their study were amplified due to the low DNA amounts present in the oil sample. Thus under these circumstances, the DNA quality becomes a more important factor than the yield to ensure that the DNA is amplifiable for downstream applications, thus avoiding any false negative detection. Therefore, Norgen’s Plant/Fungi DNA Isolation kit is capable of isolating trace amounts of DNA from processed food oils and the DNA was found to be of a PCR-amplifiable quality.
Figure 1.18S rDNA amplicons following end-point PCR of DNA samples isolated from processed food oils using Norgen’s Plant/Fungi DNA Isolation Kit. Grape DNA was isolated from leaves as a positive control. NTC = no template control.
Wheat Flour
Prior to PCR amplification, DNA samples isolated from the wheat flour were run on 1% agarose TAE gels. Unlike oil samples, sheared but still visible amounts of DNA had been isolated from the samples containing less than 20 mg flour (data not shown). However the DNA yield was not sufficient to be detected by spectrophotometer.
In the PCR amplification for the detection of plant 18S rDNA (520 bp), all DNA from the different input volumes (5mg to 50 mg) were successfully amplified. The amplification also showed that the degree of amplification increased with sample mass, indicating a high DNA quality despite the high levels of starch content in the flour samples (Figure 2). Therefore, Norgen’s Plant/Fungi DNA Isolation kit is capable of isolating trace amounts of high quality DNA from processed wheat flour.
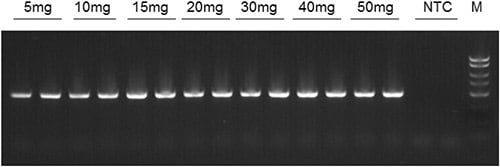
Figure 2.18S rDNA amplicons following end-point PCR of DNA samples isolated from various amounts of wheat flour using Norgen’s Plant/Fungi DNA Isolation Kit. NTC = no template control.
Conclusion
Norgen’s Plant/Fungi DNA Isolation Kit is versatile in terms of the wide array of plant samples from which it is able to isolate DNA. The kit is also sensitive in that it can isolate trace amounts of DNA, which may not be detectable visually by gel electrophoresis, but can be used for downstream applications such as PCR. This makes our kit suitable for isolating high quality DNA to be used for testing the genetic authenticity of processed food products like oils and flour which may contain altered composition and almost non-traceable amount of DNA.
References
続きを確認するには、ログインするか、新規登録が必要です。
アカウントをお持ちではありませんか?