Chiral Diene Ligands for Asymmetric Transformations
Daniel Weibel, Dr
In the last few years, a number of chiral diene ligands have emerged for a variety of asymmetric transformations.1 This powerful new method has proven to be an efficient way for the construction of enantioenriched compounds from achiral substrates. Typically, chiral ligands for late transition metals are based on nitrogen and phosphorus (e.g. BINAP, BOX), whereas chiral diene ligands (although their achiral variants are well known and important ligands for transition metal complexes) have not been exploited in asymmetric catalysis until recently when Prof. Hayashi2a and Prof. Carreira2b have published their findings.
The first effective chiral diene ligand, (R,R)-Bn-nbd*, is based on the bicyclo[2.2.1]hepta-2,5-diene core bearing benzyl groups (Scheme 1). The key step of the synthesis of (R,R)-Bn-nbd* involves a Pd-catalyzed asymmetric hydrosilylation reaction using (R)-MOP (693537) as chiral inductor followed by Tamao oxidation proceeding with good yield and excellent enantioselectivity. Further, Swern oxidation furnishes key intermediate (R,R)- bicyclo[2.2.1]heptane-2,5-dione (688959), which can be easily derivatized into the ditriflate using N-(2-Pyridyl)bis(trifluoromethanesulfonimide) (2-(NTf2)py; 403636) which undergoes Fe-catalyzed cross-coupling with benzylmagnesium chloride to give the enantiopure chiral diene ligand.3 Similarly, other C2-symmetrical chiral diene ligands such as bicyclo[2.2.2]octane-2,5-diene (bod*) and bicyclo[3.3.1]nona-2,6-diene (bnd*) are prepared via the diketone intermediate.1a
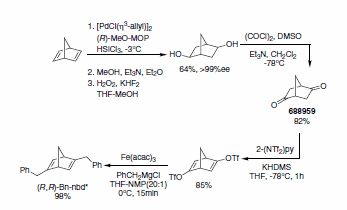
Scheme 1.
The synthetic utility of these ligands was first demonstrated in a Rh-catalyzed asymmetric 1,4-addition of aryl- and alkenylboronic acids to α,β-unsaturated ketones.
In a series of experiments, a variety of chiral diene ligands were developed and tested in this reaction2a a (PhBO)3 was used; b K3PO4 (aq.) was used instead of KOH.
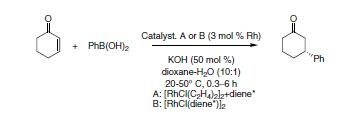
Scheme 2.
(R,R)-Ph-bod* (707171) induces high enantioselectivity in the Rh-catalyzed asymmetric 1,4-addition of arylboronic acids to α-cyanocinnamates. This method has been successfully applied to the synthesis of (R)-tolterodine (Scheme 2).4
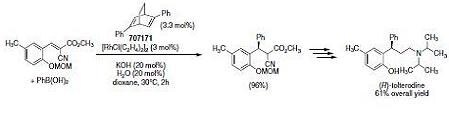
Scheme 3.
Carreira and co-workers reported the asymmetric synthesis of 3,3-diarylpropanals using a chiral bicyclo[2.2.2]octadiene derived from commercially available (R)-carvone or (S)-carvone, thus making both enantiomers readily accessible.5 This new synthesis provides access to important building blocks (Scheme 3). In a typical reaction, 3.3 mol % of the ligand and 3 mol % of the Rhodium complex in a mixture of methanol and water are required to give good yields and selectivities with both electron rich and poor boronic acids.
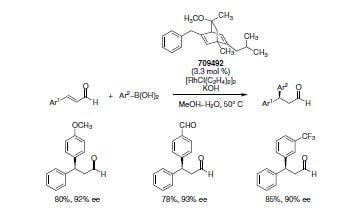
Scheme 4.
Feng and his group developed a type of chiral diene ligand based on a bicyclo[3.3.0]octadiene framework. The two cis-fused cyclopentadiene rings generate a wedge structure providing a chiral environment giving excellent enantiocontrol when coordinated to a Rhodium metal center in 1,4-addition reaction of aryl boronic acids to α,β-unsaturated carbonyl compounds6a. Similarly, 1,2-additions of aryl boronic acids to N-sulfonyl-protected arylimines provide access to enantioenriched diarylmethylamines. (Scheme 4).6b
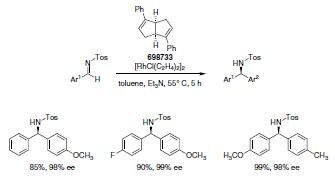
Scheme 5.
We are pleased to offer chiral diene ligands and chiral bicyclic diketone precursors for use in a variety of applications.
References
続きを確認するには、ログインするか、新規登録が必要です。
アカウントをお持ちではありませんか?