DuPhos and BPE Ligands
Introduction
Asymmetric hydrogenation reactions represent the ideal process for the commercial manufacture of single-enantiomer compounds, because of the ease by which these robust procedures can be scaled up and because of the low levels of byproducts generated in these asymmetric hydrogenations. The most effective hydrogenation systems rely on modifications of the electronic and steric properties of the ligands. Burk and co-workers succeeded in developing a highly-effective chiral phospholane class of ligands called DuPhos and BPE that contain 2,5-disubstituted groups allowing for systematic variation of the steric environment around the metal.1 We are pleased to now offer a diverse array of DuPhos and BPE phospholane ligands in collaboration with Kanata Chemical Technologies that can be ligated to metal complexes to afford highly active catalysts for asymmetric hydrogenation and other innovative transformations.2
The large-scale capacity of these robust catalysts is observed in the efficiency (substrate-to-catalyst (S/C) ratios up to 50,000) and the high activities (TOF > 5,000 h-1) in a myriad of enamide and ketone reductions. Under optimized conditions, (R,R)-Me-BPE-Rh reduced N-acetyl α-arylenamides in > 95% ee to yield valuable α-1-arylethylamines (Scheme 1).3

It should be noted that Me-DuPhos-Rh complexes were equally effective in asymmetric reductions of prochiral enamides. The general utility of these phospholane ligands is illustrated in the incredible diversity oriented production of a vast array of chiral compounds (Scheme 2).

Advantages of the DuPhos and BPE ligands:
- Superior enantiocontrol in diversity-oriented catalytic transformations
- High activities at low catalyst loadings
- Exceptional chemoselectivities for specific reaction paradigms
- Asymmetric hydrogenations of numerous unsaturated substrates
- Screening kit available to facilitate tuning selectivities
- First-to-market exclusivity for selected portfolio ligands
Representative Applications:
The reactivity profile of these innovative, chiral ligands is covered below and highlights the impressive breadth of valuable transformations mediated by the various portfolio products. In many documented cases, specific ligands have displayed unprecedented selectivities in reactions that form, for instance, chiral centers in heteroatom-functionalized organic building blocks for drug synthesis.
Asymmetric Hydrogenation of the C=N Group
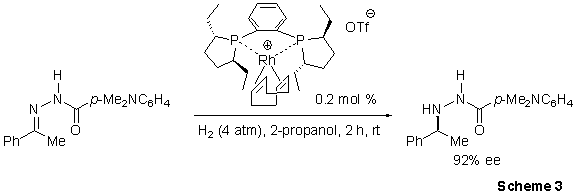
Burk and co-workers exploited the high activity of the Ethyl-DuPhos ligand via a powerful catalytic reductive amination process.4 The procedure exhibits general applicability in the reduction of a wide variety of N-aroylhydrazones, yielding enantioselectivies for most substrates > 90% (Scheme 3). Additionally this (Et-DuPhos)-Rh catalyzed system displays exceptionally high chemoselectivies, yielding little or no reduction of unfunctionalized alkenes, alkynes, ketones, aldehydes, and imines in competition experiments. The synthetic utility of these asymmetric hydrazone reductions is enhanced by their facile reaction at ambient temperature with samarium diiodide, which proceeds with no observable loss of optical purity.
Catalytic Hydrogenation of Enamides
Burk has also pioneered the asymmetric hydrogenation of various enamido olefins affording highly enantiopureδ,γ-unsaturated amino acid products.5 The (S,S)-(Et-DuPhos)-Rh catalyst system controls the reactivity of conjugated substrates with high regioselectivies as well. Under the standard hydrogenation conditions (S/C =500, H2 pressures ranging from 60 to 90 psi, and 0.5-3 h), this catalyst gave less than 2% overreduction with all products isolated in better than 95% yield. The authors elaborated upon this outstanding catalyst reactivity by demonstrating a concise and highly selective synthesis of the natural product (-)-bulgecinine, preceeded by formation of the key chiral intermediate in 99% yield with 99.3% ee (Scheme 4).

Highly Asymmetric Reductive Amidation
Burk and co-workers have also illustrated a rapid three-step process for reductive amidation, converting various ketones to the respective chiral amines with high enantioselectivity.6 Note that the transformation shown below involves reacting the ketone with hydroxylamine followed by subsequent reduction with iron metal. This methodology benefits from the utilization of crude enamides isolated as crystalline solids in the direct asymmetric catalytic hydrogenation without prior purification. The conversion of 1-acetyladamantane to the novel amine compound occurs with >99% ee and in high yield via use of cationic Rh(I) catalyst 1 (Scheme 5).

Preparation of Chiral Organics with C-O Stereogenic Centers

Neil Boaz has also utilized rhodium(I)-((R,R)-Me-DuPhos) catalyst 1 to produce chiral alcohols via the asymmetric hydrogenation of enol esters. Allylic alcohol derivatives are desirable organic building blocks in diversity-oriented synthesis, because the olefin can be further functionalized after the stereochemistry has been set in the hydrogenation.7 Under asymmetric hydrogenation conditions, the initially formed propargylic acetate was subsequently reduced to yield the Z-allylic acetate. Impressively the enantioselectivity observed in this reaction was very high among the general substrate class 2a-c.

Burk and co-workers have also designed highly effective catalysts for the asymmetric reduction of C=O bonds under hydrogenation conditions.8 In the case below, the methodology proceeded via use of a chiral Ru(II)Br2-(i-Pr-BPE) complex, which was prepared by reacting [(COD)Ru(2-methylallyl)2] with the BPE ligand followed by treatment with methanolic HBr. A variety of esters were rapidly hydrogenated as mediated by this catalyst to the hydroxyl esters with very high enantioselectivities ranging from > 98% ee for the alkyl-substituted substrates (cf. Scheme 7).

Preparation of Chiral Organics with C-C Stereogenic Centers
As a complement to the C-O bond-forming asymmetric synthesis, Burk and co-workers have performed highly enantioselective hydrogenations of unique substrates that are precursors to natural products. This methodology is highlighted by enantioselectivies approaching 100% and high efficiencies (S/C > 2500), and has allowed this research team to surmount the problems associated with the asymmetric synthesis of the drug candoxitril (Scheme 8).9
Novel Asymmetric [4+1] Cycloadditions
These highly practical enantioselective reactions have been accomplished by the Murkami research group, thus expanding the synthetic utility of these powerful chiral ligands.10 The [4+1] cycloaddition between vinylallenes and carbon monoxide affords complex cyclopentenone derivatives in a single step and with enantiopurities up to 95% (Scheme 9).

Palladium-Catalyzed Asymmetric Phosphination
Glueck and co-workers have successfully developed a catalytic asymmetric phosphination reaction utilized in the production of P-chirogenic phosphines(Scheme 10).11 The fast conversions, moderate catalyst loadings, and enantioselectivies allow for the expeditious construction of enantioenriched phosphine building blocks. Metal-catalyzed routes to these valuable P-chiral ligands are rare making this methodology attractive as a viable route to synthesize other phosphine ligands.

Catalytic Asymmetric Alkylation of N-Diphenylphosphinoylimines
Recently Charette has published a facile asymmetric synthesis of various a-chiral amines via the enantioselective addition of dialkylzinc reagents to N-phosphinoylimines.12 (R,R)-Me-DuPhos, in conjunction with a Cu(OTf)2 source, yielded exceptional enantiomeric excess for this reaction paradigm above 90% for all aromatic substrates (Scheme 11). The mild reaction conditions, reasonable temperatures, and use of various dialkylzinc reagents all contribute to the attractiveness of this methodology.

Conclusion
The modular nature of these chiral phospholane ligands has allowed variation of the electronics and sterics around the metal upon complexation. These bidentate ligands perform highly enantioselective hydrogenation reactions to produce a wide array of compounds containing C-C, C-O, and C-N stereogenic centers. We are your dedicated source for a broad spectrum of building blocks that provide essential starting materials in the synthesis of complex organic molecules. Our growing portfolio of catalysis products, supplemented by the DuPhos/BPE family, strongly complements our existing chemical line and will accelerate your research success.
References
続きを確認するには、ログインするか、新規登録が必要です。
アカウントをお持ちではありませんか?