O9515
Prolyl oligopeptidase
recombinant, expressed in E. coli
Synonym(s):
Prolyl endopeptidase
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
UNSPSC Code:
12352204
NACRES:
NA.54
Recommended Products
recombinant
expressed in E. coli
Quality Level
Assay
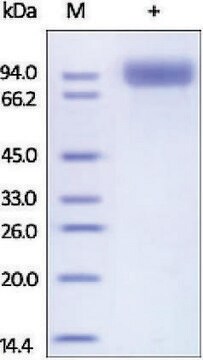
≥90% (SDS-PAGE)
form
solution
specific activity
≥10 unit/μg protein
mol wt
81.6 kDa
shipped in
dry ice
storage temp.
−70°C
General description
Prolyl oligopeptidase (PO) is mapped to human chromosome 6q22. It comprises of a N-terminal β-propeller domain and C-terminal α/β hydrolase catalytic domain. PO is highly expressed in the neuronal cytoplasm and its expression increases with age.
Prolyl oligopeptidase is a cytosolic serine peptidase which cleaves peptide bonds at the C′ terminal side of prolines. It is only capable of processing peptides containing no more than 30 amino acids due to the unique β-propeller region that regulates access to the active site.
Application
Prolyl oligopeptidase has been used in a study to assess the mechanism of lithium ion action. It has also been used in a study to investigate its distribution in human tissue and body fluids.
Prolyl oligopeptidase has been used in the prolyl oligopeptidase inhibitory activity assay in lyophilized protein hydrolysate samples and Schistosoma mansoni samples.
Biochem/physiol Actions
Prolyl oligopeptidase (PO) regulates physiological processes and is crucial for the generation of active hormone and peptide fragments from their precursors. It shows elevated levels in psychiatric disorders and Alzheimer′s patients. Altered levels of PO is observed in patients with bipolar disorder. High expression of PO may promote metastasis of malignant ovarian and colorectal tumors.
Unit Definition
One unit will hydrolyze 1.0 picomole of Ala-Pro-aminomethylcoumarin per minute at pH 7.4 at 25 °C.
Physical form
Supplied as a solution in 45 mM Tris-HCl, pH 8.0, 124 mM NaCl, 2.4 mM KCl, 10% glycerol, 3 mM DTT and variable amounts of imidazole.
Preparation Note
N-terminal GST-tagged 81.6 kDa full-length protein
Storage Class Code
12 - Non Combustible Liquids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Structure-function properties of prolyl oligopeptidase family enzymes
Rea D and Fulop V
Cell Biochemistry and Biophysics, 44(3), 349-365 (2006)
R S Williams et al.
The EMBO journal, 18(10), 2734-2745 (1999-05-18)
The therapeutic properties of lithium ions (Li+) are well known; however, the mechanism of their action remains unclear. To investigate this problem, we have isolated Li+-resistant mutants from Dictyostelium. Here, we describe the analysis of one of these mutants. This
Distribution of prolyl oligopeptidase in human peripheral tissues and in ovarian and colorectal tumors
Myohanen TT, et al.
The Journal of Histochemistry and Cytochemistry, 60(9), 706-715 (2012)
Prolyl oligopeptidase and bipolar disorder
Williams, Robin SB
Clinical neuroscience research, 4(3-4), 233-242 (2004)
L Polgár
Cellular and molecular life sciences : CMLS, 59(2), 349-362 (2002-03-28)
A group of serine peptidases, the prolyl oligopeptidase family, cannot hydrolyze peptides containing more than about 30 residues. This group is unrelated to the classical trypsin and subtilisin families, and includes dipeptidyl peptidase IV, acylaminoacyl peptidase and oligopeptidase B, in
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service