Aldehydes as Building Blocks
Hartmann and co-workers have described the synthesis of a series of potent and selective inhibitors of aldosterone synthase (CYP11B2), in which the key synthetic step was a Wittig reaction using various heterocyclic aldehydes (Scheme 1). The isoquinoline adduct was a potent and selective inhibitor of CYP11B2. The successful inhibition of CYP11B2 has been proposed as a strategy for treatment of congestive heart failure and myocardial fibrosis.1
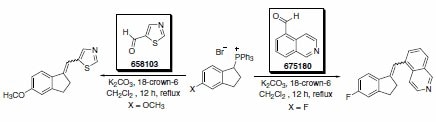
Scheme 1. Product No. 658103 and 675180
4-(1-Pyrrolidino)benzaldehyde was reported as a key building block in the synthesis of an improved inhibitor of NS5B polymerase of the hepatitis C virus (Scheme 2).2 The product from this substrate displayed significant improvement in the potency of the original HTS lead compound (Figure 1).
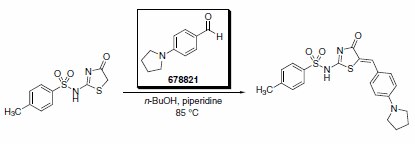
Scheme 2.Product No. 678821
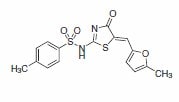
Figure 1.
Professor Jørgensen’s group reported an asymmetric formal synthesis of (–)-paroxetine, a selective serotonin reuptake inhibitor used in the treatment of anxiety and other psychological disorders.3 The key step involved the organocatalytic conjugate addition of dibenzyl malonate to trans-4-fluorocinnamaldehyde (Scheme 3). Similarly, Wang’s research group has used the Jørgensen organocatalyst in a tandem Michael-aldol reaction to furnish chiral thiochromenes in excellent yields (Scheme 4).4
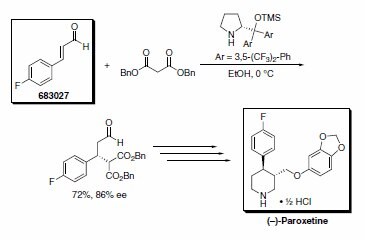
Scheme 3. Product No. 683027
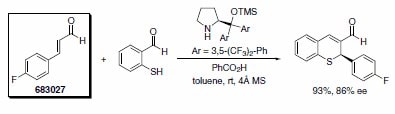
Scheme 4. Product No. 683027
References
Per continuare a leggere, autenticati o crea un account.
Non hai un Account?