10280879001
Roche
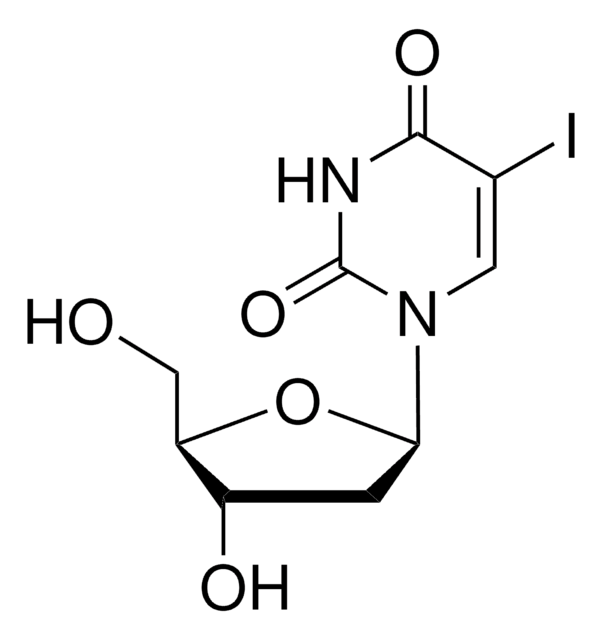
5-Bromo-2′-deoxyuridine
>97%, crystalline solid, pkg of 1 g
Synonym(s):
5-Bromo-2′-deoxyuridine, brdu, 5-BrdU, 5-Bromo-1-(2-deoxy-β-D-ribofuranosyl)uracil, 5-Bromouracil deoxyriboside, BUdR
About This Item
Recommended Products
Quality Level
Assay
>97%
form
crystalline solid
mol wt
307.1
packaging
pkg of 1 g
manufacturer/tradename
Roche
mp
191-194 °C (dec.) (lit.)
shipped in
wet ice
storage temp.
2-8°C
SMILES string
OC[C@H]1O[C@H](C[C@@H]1O)N2C=C(Br)C(=O)NC2=O
InChI
1S/C9H11BrN2O5/c10-4-2-12(9(16)11-8(4)15)7-1-5(14)6(3-13)17-7/h2,5-7,13-14H,1,3H2,(H,11,15,16)/t5-,6+,7+/m0/s1
InChI key
WOVKYSAHUYNSMH-RRKCRQDMSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
Biochem/physiol Actions
Quality
Preparation Note
According to literature, 5 to 50 mg/kg body weight mice have been used for the detection of cell proliferation.
Preparation of Working Solution
Corresponding to the BrdU-labeling reagent which is included in the Roche Cell Proliferation ELISA, BrdU kits and in the Roche 5-Bromo-2′-deoxy-uridine Labeling and Detection Kits, prepare the BrdU working solution by dissolving the substance in PBS to a 10 mM stock solution (MW of BrdU = 307.1 D). For the in vivo use of BrdU dissolve in PBS; for the in vitro use of BrdU, dissolve in double-distilled water at the same 10 mM concentration.
Storage conditions (working solution): -15 to -25 °C
Other Notes
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Muta. 1B - Repr. 2
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service