P6994
Phenol nitroprusside solution
Synonym(s):
Nitroprusside Phenol Solution
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
UNSPSC Code:
12352401
NACRES:
NA.25
Recommended Products
reaction suitability
reagent type: oxidant
storage temp.
2-8°C
Related Categories
Application
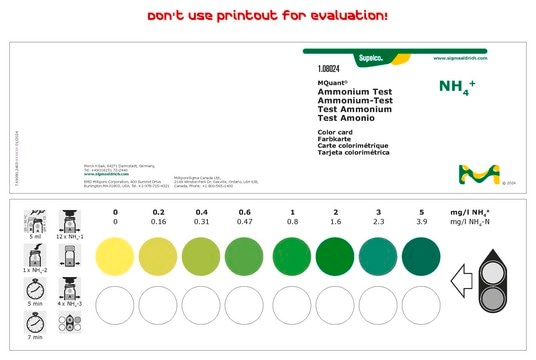
Suitable for the colorimetric determination of ammonia using alkaline hypochlorite (A1727) solution as used in urea nitrogen assays
Other Notes
Contains sodium nitroprusside, phenol and EDTA.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Chronic 3 - Eye Dam. 1 - Muta. 2 - Skin Corr. 1B
Storage Class Code
6.1D - Non-combustible acute toxic Cat.3 / toxic hazardous materials or hazardous materials causing chronic effects
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
An improved method for the estimation of ammonia in blood plasma.
D B Horn et al.
Clinica chimica acta; international journal of clinical chemistry, 17(1), 99-105 (1967-07-01)
Functionalized self-assembling peptide hydrogel enhance maintenance of hepatocyte activity in vitro.
Elsa Genové et al.
Journal of cellular and molecular medicine, 13(9B), 3387-3397 (2009-11-17)
There is a major challenge in maintaining functional hepatocytes in vivo as these cells rapidly lose their metabolic properties in culture. In this work we have developed a bioengineered platform that replaces the use of the collagen I--in the traditional
Alberto Amaretti et al.
Frontiers in microbiology, 10, 2614-2614 (2019-12-06)
Unabsorbed proteins reach the colon and are fermented by the microbiota, yielding a variety of harmful metabolites. In the present study, a 16S rRNA gene survey identified the bacterial taxa flourishing in 11 batch fermentations with proteins and peptones as
Suzanne Z Andersen et al.
Nature, 570(7762), 504-508 (2019-05-23)
The electrochemical synthesis of ammonia from nitrogen under mild conditions using renewable electricity is an attractive alternative1-4 to the energy-intensive Haber-Bosch process, which dominates industrial ammonia production. However, there are considerable scientific and technical challenges5,6 facing the electrochemical alternative, and
Byung Hee Ko et al.
Nature communications, 11(1), 5856-5856 (2020-11-19)
The electroreduction of carbon dioxide offers a promising avenue to produce valuable fuels and chemicals using greenhouse gas carbon dioxide as the carbon feedstock. Because industrial carbon dioxide point sources often contain numerous contaminants, such as nitrogen oxides, understanding the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service