All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C40H36F10IrN4P
CAS Number:
Molecular Weight:
985.92
UNSPSC Code:
51171641
NACRES:
NA.23
Recommended Products
form
powder or crystals
Quality Level
reaction suitability
core: iridium
reagent type: catalyst
reaction type: Photocatalysis
mp
>300 °C
photocatalyst activation
465 nm
Application
This photocatalyst readily facilitates alkynylation of carboxylic acids as well as the decarboxylative cross-coupling of oxalates via metallaphotoredox catalysis.
Product can be used with our line of photoreactors: Including Penn PhD (Z744035) & SynLED 2.0 (Z744080)
Product can be used with our line of photoreactors: Including Penn PhD (Z744035) & SynLED 2.0 (Z744080)
related product
Product No.
Description
Pricing
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Timothy M Monos et al.
The Journal of organic chemistry, 81(16), 6988-6994 (2016-06-16)
We report a rapid, one-pot, operationally simple, and scalable preparation of valuable cationic heteroleptic iridium(III) polypyridyl photosensitizers. This method takes advantage of two consecutive microwave irradiation steps in the same reactor vial, avoiding the need for additional reaction purifications. A
Xiaheng Zhang et al.
Journal of the American Chemical Society (2016-10-11)
Alkyl oxalates, prepared from their corresponding alcohols, are engaged for the first time as carbon radical fragments in metallaphotoredox catalysis. In this report, we demonstrate that alcohols, native organic functional groups, can be readily activated with simple oxalyl chloride to
Strongly Blue Luminescent Cationic Iridium(III) Complexes with an Electron-Rich Ancillary Ligand: Evaluation of Their Optoelectronic and Electrochemiluminescence Properties.
Ladouceur S,et al.
European Journal of Inorganic Chemistry, 2013, 5329-5343 (2013)
Franck Le Vaillant et al.
Angewandte Chemie (International ed. in English), 54(38), 11200-11204 (2015-07-28)
Alkynes are used as building blocks in synthetic and medicinal chemistry, chemical biology, and materials science. Therefore, efficient methods for their synthesis are the subject of intensive research. Herein, we report the direct synthesis of alkynes from readily available carboxylic
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service![(Ir[dF(CF3)ppy]2(dtbpy))PF6](/deepweb/assets/sigmaaldrich/product/structures/982/913/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09/640/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09.png)
![[Ir(dtbbpy)(ppy)2]PF6](/deepweb/assets/sigmaaldrich/product/structures/158/329/2544d673-d267-4aa1-8f46-2652aad4bfa0/640/2544d673-d267-4aa1-8f46-2652aad4bfa0.png)
![[Ir(p-F(Me)ppy)2-(4,4′-dtbbpy)]PF6 ≥95%](/deepweb/assets/sigmaaldrich/product/structures/231/079/a5445824-9d4b-4c84-9c5f-f3acbcc75fd4/640/a5445824-9d4b-4c84-9c5f-f3acbcc75fd4.png)
![[Ir(dF(Me)ppy)2(dtbbpy)]PF6](/deepweb/assets/sigmaaldrich/product/structures/150/099/7c2dfa31-39f4-4cca-aee5-86d4a89fea78/640/7c2dfa31-39f4-4cca-aee5-86d4a89fea78.png)
![[Ir(dFCF3ppy)2-(5,5’-dCF3bpy)]PF6 ≥95%](/deepweb/assets/sigmaaldrich/product/structures/422/901/e00f3148-fb86-4f94-9e79-21d064c3f327/640/e00f3148-fb86-4f94-9e79-21d064c3f327.png)
![[Ir{dFCF3ppy}2(bpy)]PF6](/deepweb/assets/sigmaaldrich/product/structures/180/924/79119ac4-7d62-429d-b23d-a14c012c6050/640/79119ac4-7d62-429d-b23d-a14c012c6050.png)

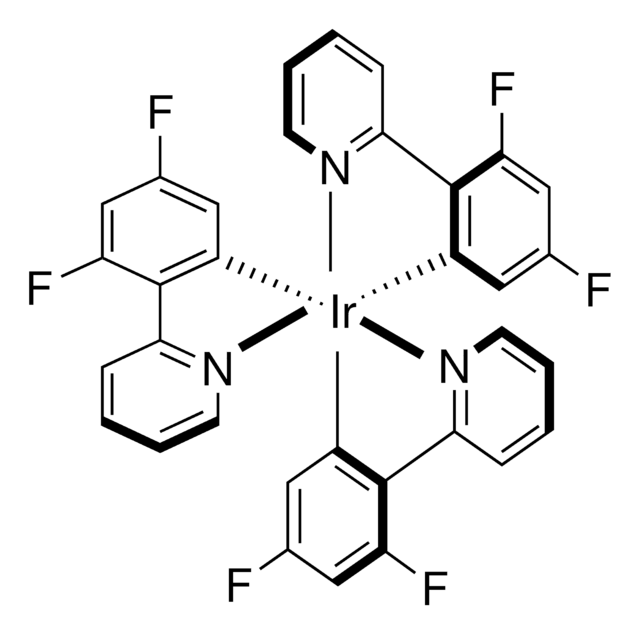


![Tris[2-(4,6-difluorophenyl)pyridinato-C2,N]iridium(III) 96%](/deepweb/assets/sigmaaldrich/product/structures/299/364/88650481-ef29-49a1-a324-7b3e305d12be/640/88650481-ef29-49a1-a324-7b3e305d12be.png)
![Ir[dF(t-Bu)-ppy]3](/deepweb/assets/sigmaaldrich/product/structures/254/294/d0fb19e5-05b2-4c1b-990b-a99fa60b3e73/640/d0fb19e5-05b2-4c1b-990b-a99fa60b3e73.png)

![(Ir[Me(Me)ppy]2(dtbpy))PF6](/deepweb/assets/sigmaaldrich/product/structures/649/610/87d2ceeb-25e4-485a-ac33-8ebf819c22dd/640/87d2ceeb-25e4-485a-ac33-8ebf819c22dd.png)
![Dichlorotetrakis[3,5-difluoro-2-(2-pyridinyl)phenyl]diiridium(III) 95%](/deepweb/assets/sigmaaldrich/product/structures/300/702/f03c66ed-f8fa-4103-a508-e57483592685/640/f03c66ed-f8fa-4103-a508-e57483592685.png)
![Ir[p-F(t-Bu)-ppy]3](/deepweb/assets/sigmaaldrich/product/structures/189/186/7badaac3-82af-4109-aab5-dea3a3aa916d/640/7badaac3-82af-4109-aab5-dea3a3aa916d.png)
![Tris[2-phenylpyridinato-C2,N]iridium(III) 97%](/deepweb/assets/sigmaaldrich/product/structures/167/234/658d0b76-d31d-4fd5-8041-e04e207227c9/640/658d0b76-d31d-4fd5-8041-e04e207227c9.png)