Identification of Fixed Oils by Thin layer chromatography (Method II, USP Monograph)
Sanjay Poman, R&D Scientist
Introduction
Vegetable oil and fats are integral components of our diet. They are used in different ways such as cooking or frying oils, salad dressings, and as ingredients in food product formulations. They are important from both nutritional and economic perspectives. However, ensuring their authenticity has been a critical issue since the old times The food industry has an important need to authenticate these oils and fats and ensure their quality.
Several methods have been employed to check the purity of edible oils and fats. As each oil and fat may have special components at known levels, their presence and concentrations may be used as a detection tool.
High-performance thin-layer chromatography (HPTLC) is a fast and efficient fingerprinting method for the identification of fixed oils by thin-layer chromatography, following USP 43 NF 38 monograph method containing USP general chapter <202>.1 The USP Identification test for fixed oils uses a suitable octadecyl silyl silica gel as the phase for high-performance thin-layer chromatography (HPTLC).
This application note illustrates the use of USP general chapter <202> for the identification of fixed oils by thin-layer chromatography, which includes the HPTLC method for the identification of different fatty oils.
EXPERIMENTAL CONDITIONS | |
|---|---|
Plate: | Silica Gel 60 RP18 F254s 20 x 10 cm HPTLC plate (1.16225) |
Application volume: | 2 µL bands of 8 mm |
Immersion reagent: | 25 mg/mL of Phosphomolybdic acid in 96% alcohol |
Chamber: | Condition the plate to relative humidity of 33% |
Mobile phase: | Methylene chloride:glacial acetic acid:acetone (20:40:50) (v/v/v) |
Plate development: | Prewash the plate in methylene chloride and allow it to dry the plate at 120˚C for 10 minutes. Sample and standard solutions are applied as bands with a bandwidth of 8 mm, using a migration distance of 7 cm, tracks were applied to the HPTLC plates are from left to right. |
Migration distance: | 7 cm |
Drying: | After development of the chromatogram allow the plate to dry in air, treat it with an immersion reagent and heat the plate at 120˚C for 3 minutes, cool and examine the plate in white light. |
Sample diluent: | Methylene chloride |
Standard solution: | Dissolve 25 µL of the appropriate USP reference standard in 3 mL of methylene chloride |
Sample solution: | Dissolve 25 µL of the fixed oil samples in 3 mL of methylene chloride |
SST solution 1: | Dissolve 25 µL of USP corn oil RS in 3 mL of methylene chloride |
SST Solution 2: | Dissolve 25 µL of USP olive oil RS in 3 mL of methylene chloride |

Figure 1.A developed HPTLC plate for HPTLC analysis of fixed oil samples. Tracks: 1. Olive oil USP RS, 2. Corn oil USP RS, 3. Almond oil, 4. Groundnut oil, 5. Sunflower oil, 6. Coconut oil
Chromatographic Data -System Suitability Test Solutions (SST)

Figure 2.HPTLC bands of olive (left) and corn oil (right) in fixed oil samples.
Track No | Intense Spot Number | Oil standard | Rf |
|---|---|---|---|
1 | 1 | Olive oil USP RS | 0.28 |
2 | Olive oil USP RS | 0.32 | |
2 | 1 | Corn oil USP RS | 0.28 |
2 | Corn oil USP RS | 0.32 | |
3 | Corn oil USP RS | 0.38 | |
4 | Corn oil USP RS | 0.42 |
System suitability criteria
Four principal spots from corn and the two principal bands from olive oil are clearly identified and separated.
Acceptance Criteria
Rf values of the principal bands in the sample solutions correspond to those of the standard solution.
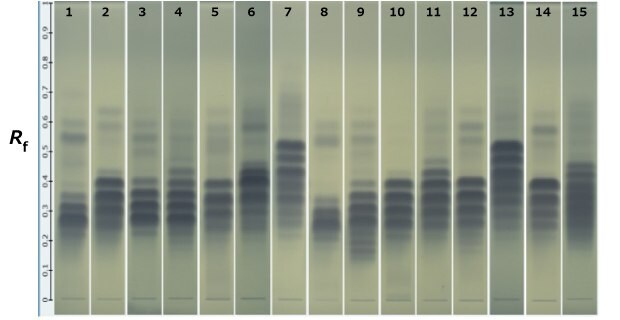
Figure 3.HPTLC bands of different oils.
Track | Sample |
|---|---|
1 | Olive Oil standard |
2 | Corn Oil standard |
3 | Almond Oil |
4 | Canola Oil |
5 | Cotton seed Oil |
6 | Evening primerose Oil |
7 | Flax Seed Oil |
8 | Palm Oil |
9 | Groundnut Oil |
10 | Sesame Oil |
11 | Soyabean 0il |
12 | Sunflower Oil |
13 | Chia Oil |
14 | Safflower Oil |
15 | Borage Oil |
Conclusion
Using HPTLC plates and following the method prescribed in the USP 43 NF38 monograph using USP general chapter <202> guidelines, the acceptance criteria for fixed oil under the identification test was met for the market samples. 13 various market samples of oils were tested as per the procedure, and it was found that the oils contain well-separated bands having similar Rf values to that of standard bands in the standard oil. A separate study of almond, groundnut, sunflower, and coconut oils shows well-separated bands having similar Rf values to that of standard olive oil and corn oil standards meeting the USP monograph method acceptance criteria as well as SST criteria.
To help analytical labs test for essential oil adulteration, suitable analytical standards of common chemical markers used in essential oils authenticity testing are listed in the below product list.
References
To continue reading please sign in or create an account.
Don't Have An Account?