52927
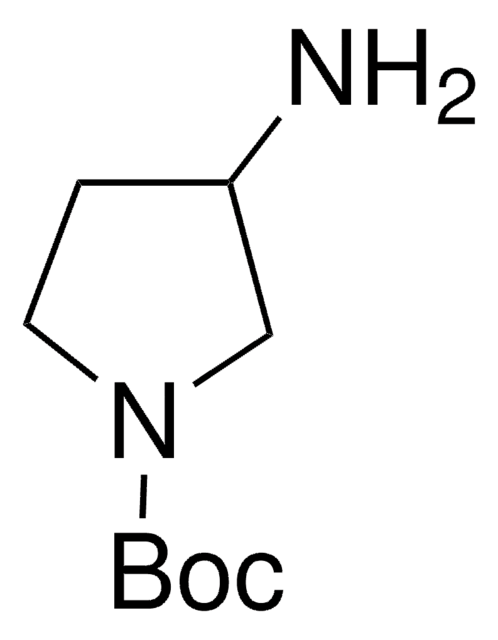
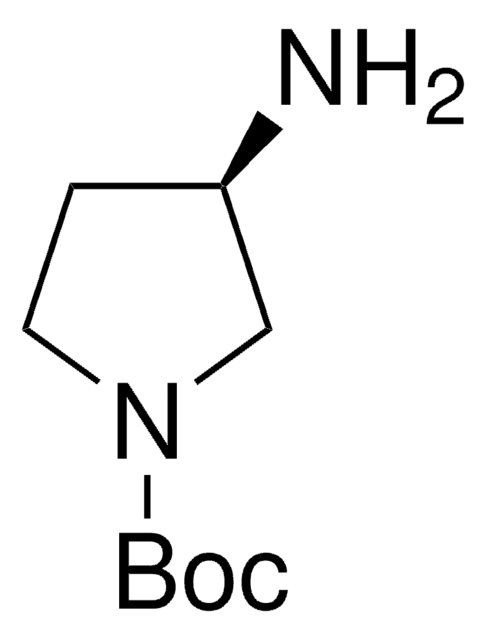
(S)-3-(Boc-amino)pyrrolidine
≥98.0% (TLC)
Synonym(s):
tert-Butyl (S)-3-pyrrolidinylcarbamate
About This Item
Recommended Products
Assay
≥98.0% (TLC)
form
solid
optical activity
[α]/D -21.5±2.0°, c = 1 in ethanol
SMILES string
CC(C)(C)OC(=O)N[C@H]1CCNC1
InChI
1S/C9H18N2O2/c1-9(2,3)13-8(12)11-7-4-5-10-6-7/h7,10H,4-6H2,1-3H3,(H,11,12)/t7-/m0/s1
InChI key
DQQJBEAXSOOCPG-ZETCQYMHSA-N
Application
- 2,4,6-trisubstitued pyrido[3,4-d]pyrimidine derivatives as potent inhibitors against EGFR tyrosine kinase.
- Aminopyrrolidine scaffolds for asymmetric Morita−Baylis-Hillman reaction.
- N-benzyl-3-sulfonamidopyrrolidines as potent bacterial cell division inhibitors.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
Mono-Boc-protected diamines are versatile building blocks for chemical synthesis. Their production is a lot more challenging than the simple reaction scheme might imply, because the Boc-anhydride reagent cannot differentiate between the two identical amino moieties in the substrate.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service