39303
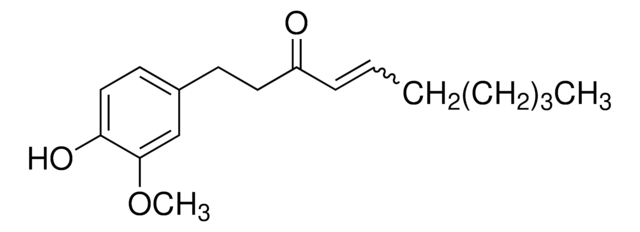
[6]-Shogaol
analytical standard
Synonym(s):
1-(4-Hydroxy-3-methoxyphenyl)-4-decen-3-one
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C17H24O3
CAS Number:
Molecular Weight:
276.37
Beilstein:
2056098
MDL number:
UNSPSC Code:
85151701
PubChem Substance ID:
NACRES:
NA.24
Recommended Products
grade
analytical standard
Quality Level
Assay
≥90.0% (HPLC)
shelf life
limited shelf life, expiry date on the label
technique(s)
HPLC: suitable
gas chromatography (GC): suitable
application(s)
food and beverages
format
neat
storage temp.
2-8°C
SMILES string
CCCCC\C=C\C(=O)CCc1ccc(O)c(OC)c1
InChI
1S/C17H24O3/c1-3-4-5-6-7-8-15(18)11-9-14-10-12-16(19)17(13-14)20-2/h7-8,10,12-13,19H,3-6,9,11H2,1-2H3/b8-7+
InChI key
OQWKEEOHDMUXEO-BQYQJAHWSA-N
Looking for similar products? Visit Product Comparison Guide
General description
[6]-Shogaol is a pungent and one of the most abundant dehydrated form of gingerols present in the fresh ginger roots or dried and thermally treated roots. It may serve as a potential anti-inflammatory agent.
Application
Refer to the product′s Certificate of Analysis for more information on a suitable instrument technique. Contact Technical Service for further support.
Packaging
Bottomless glass bottle. Contents are inside inserted fused cone.
Other Notes
This compound is commonly found in plants of the genus: zingiber
Recommended products
Find a digital Reference Material for this product available on our online platform ChemisTwin® for NMR. You can use this digital equivalent on ChemisTwin® for your sample identity confirmation and compound quantification (with digital external standard). An NMR spectrum of this substance can be viewed and an online comparison against your sample can be performed with a few mouseclicks. Learn more here and start your free trial.
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Sehwan Shim et al.
Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association, 50(3-4), 597-602 (2011-12-07)
[6]-Shogaol has beneficial effects in spinal neuronal regeneration, but associated molecules and mechanisms are not identified. Neurotrophic factors, including brain-derived neurotrophic factor (BDNF), are associated with proliferation and differentiation of neuronal cells and exert a neuroprotective effect in neurodegenerative models.
Qingliang Qiao et al.
Journal of chromatography. A, 1218(36), 6187-6190 (2011-01-05)
The flash high speed counter-current chromatographic (FHSCCC) separation of gingerols and 6-shogaol was performed on a HSCCC instrument equipped with a 1200-ml column (5 mm tubing i.d.) at a flow rate of 25 ml/min. The performance met the FHSCCC feature
Min-Ji Bak et al.
Molecules (Basel, Switzerland), 17(7), 8037-8055 (2012-07-06)
The rhizome of ginger (Zingiber officinale Roscoe) is known to have several bioactive compounds including gingerols and shogaols which possess beneficial health properties such as anti-inflammatory and chemopreventive effects. Based on recent observations that 6-shogaol may have more potent bioactivity
6-Shogaol reduced chronic inflammatory response in the knees of rats treated with complete Freund's adjuvant
Levy SAA, et al.
BioMed Central Pharmacology, 6, 12-12 (2006)
Yingdong Zhu et al.
PloS one, 8(1), e54677-e54677 (2013-02-06)
Our previous study found that [6]-shogaol, a major bioactive component in ginger, is extensively metabolized in cancer cells and in mice. It is unclear whether these metabolites retain bioactivity. The aim of the current study is to synthesize the major
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service![[6]-Gingerol analytical standard](/deepweb/assets/sigmaaldrich/product/structures/259/444/6877889c-1cc0-47f5-b807-f847deadec1d/640/6877889c-1cc0-47f5-b807-f847deadec1d.png)
![[6]-Gingerol, Zingiber officinale An antitumor, apoptosis-inducing compound of the ginger family that blocks EGF-induced cell transformation by inhibiting the activation of Activator Protein-1 (AP-1).](/deepweb/assets/sigmaaldrich/product/images/140/919/0846df46-0b67-4c28-b99d-87e177be65b2/640/0846df46-0b67-4c28-b99d-87e177be65b2.jpg)
![[10]-Gingerol ≥98% (HPLC)](/deepweb/assets/sigmaaldrich/product/structures/224/210/b4f3e699-03b9-4112-89c1-a63f196344d0/640/b4f3e699-03b9-4112-89c1-a63f196344d0.png)
![[8]-Gingerol analytical standard](/deepweb/assets/sigmaaldrich/product/structures/408/530/af2f2837-3419-4e07-a72b-24e95af0d7ce/640/af2f2837-3419-4e07-a72b-24e95af0d7ce.png)