All Photos(1)
About This Item
Linear Formula:
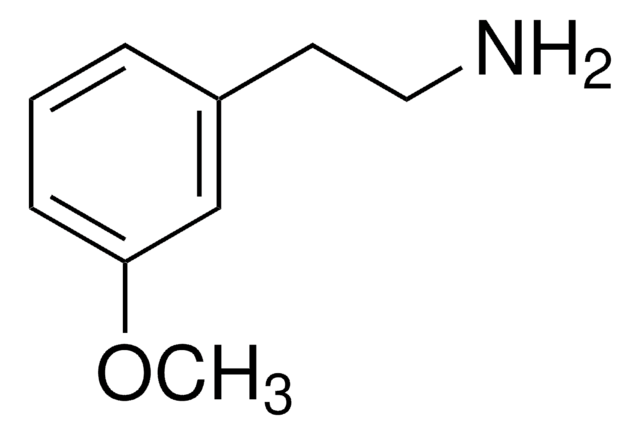
(CH3O)2C6H3CH2CH2NH2
CAS Number:
Molecular Weight:
181.23
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
Recommended Products
Assay
97%
drug control
USDEA Schedule I
refractive index
n20/D 1.5320 (lit.)
bp
261-262 °C (lit.)
density
1.178 g/mL
SMILES string
COc1cccc(CCN)c1OC
InChI
1S/C10H15NO2/c1-12-9-5-3-4-8(6-7-11)10(9)13-2/h3-5H,6-7,11H2,1-2H3
InChI key
XKBUFTXNLBWTFP-UHFFFAOYSA-N
General description
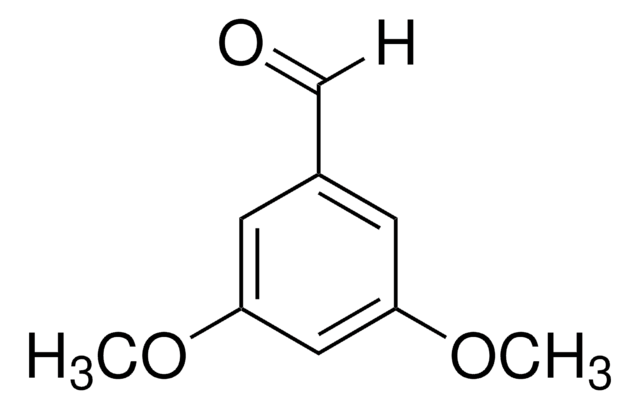
2,3-Dimethoxyphenethylamine is identified as one of the components in bamboo (Phyllostachys pubescens) plant extract by gas chromatographic- mass spectrometric analysis. 1-(2,3-dimethoxyphenyl)-2-nitroethene undergoes reduction reaction with lithium aluminum hydride (LiAlH4) in the presence of dry tetrahydrofuran to yield 2,3-dimethoxyphenethylamine.
Application
2,3-Dimethoxyphenethylamine may be used in the synthesis of N-(2,3-dimethoxyphenethyl)-2-(3,4-dimethoxyphenyl)acetamide and N-(3,4-dimethoxyphenethyl)-phenyl acetamide.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8B - Non-combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Gas chromatography-mass spectrometry analysis and chemical composition of the bamboo-carbonized liquid.
Bilehal D, et al.
Food Analytical Methods, 5(1), 109-112 (2012)
Tetramethoxydibenzoquinolizinium salts. Preparation and antileukemic activity of some positional and structural isomers of coralyne.
Zee-Cheng RKY and Cheng CC.
Journal of Medicinal Chemistry, 19(7), 882-886 (1976)
Chen-Yuan Kuo et al.
European journal of medicinal chemistry, 44(3), 1271-1277 (2008-10-15)
A series of phenoxyisoquinolines, N-phenoxyethyl-1-(2-nitrophenyl)-1,2,3,4-THIQs 3a-3d, N-phenoxyethyl-1-benzyl-1,2,3,4-THIQ 3e, N-phenoxyethyl-1-(2-aminophenyl)-1,2,3,4-THIQs 5f-5i, N-phenoxyethyl-1-(2-phenoxyethylaminophenyl)-1,2,3,4-THIQs 5f'-5i', have been synthesized and tested in isolated rat vas deferens alpha-adrenoreceptors. Comparison of pA2 values for these compounds in the presence of phenylephrine confirms that alpha(1)-adrenoceptor blocking activity
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service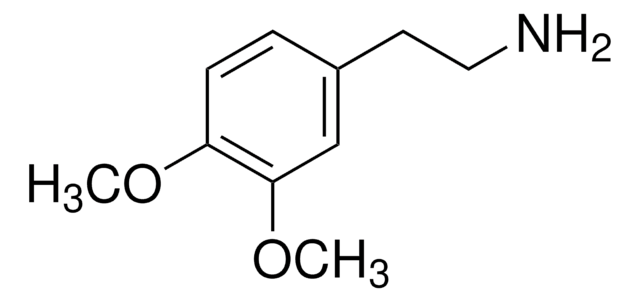



![1,5,7-Triazabicyclo[4.4.0]dec-5-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/171/446/333d560c-cff6-4958-b489-5acfb3057cce/640/333d560c-cff6-4958-b489-5acfb3057cce.png)
