All Photos(1)
About This Item
Linear Formula:
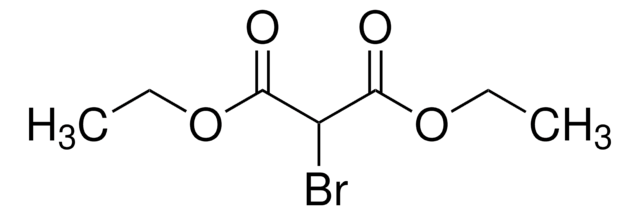
Br2C(CO2C2H5)2
CAS Number:
Molecular Weight:
317.96
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
liquid
refractive index
n20/D 1.484 (lit.)
bp
140-143 °C/18 mmHg (lit.)
density
1.68 g/mL at 25 °C (lit.)
SMILES string
CCOC(=O)C(Br)(Br)C(=O)OCC
InChI
1S/C7H10Br2O4/c1-3-12-5(10)7(8,9)6(11)13-4-2/h3-4H2,1-2H3
InChI key
PFZYFZRUPFUEOB-UHFFFAOYSA-N
General description
Diethyl dibromomalonate reacts with sodium methoxide in cyclohexene to afford dibromonorcarane. It also reacts with allyl(pyridine)cobaloximes to afford the corresponding allyl-substituted esters.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Investigations into the Bromination of Substituted Phenols using Diethyl Bromomalonate and Diethyl Dibromomalonate.
Coumbarides GS, et al.
Bulletin of the Chemical Society of Japan, 74(1), 179-180 (2001)
Reactions of organocobalt complexes with bromoesters: regiospecific synthesis of allyl-and cyclopropylmethyl-substituted malonic and acetoacetic esters.
Veber M, et al.
Journal of Organometallic Chemistry, 209(3), 393-399 (1981)
Reaction of diethyl dibromomalonate with methoxide: Evidence for a novel bromophilic attack.
Mebane RC, et al.
Tetrahedron Letters, 40(8), 1459-1462 (1999)
Dominik Schuch et al.
Journal of the American Chemical Society, 131(36), 12918-12920 (2009-08-22)
Tetrahydrofur-2-ylmethyl radicals were stereoselectively generated from substituted pent-4-en-1-ols in aerobic cobalt(II)-catalyzed oxidations. Intermediates were trapped with cyclohexa-1,4-diene, gamma-terpinene, BrCCl(3), diethyl dibromomalonate, or electron-deficient olefins such as acrylonitrile or dimethyl fumarate to afford functionalized tetrahydrofurans in synthetically useful yields.
A novel bromination for an unsaturated a-anion ester. Synthesis of 2-bromo-cis-8, cis-11, cis-14-eicosatrienoic acid.
van der Wolf L and Pabon HJJ.
Rec. Trav. Chim., 96(3), 72-74 (1977)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service