584222
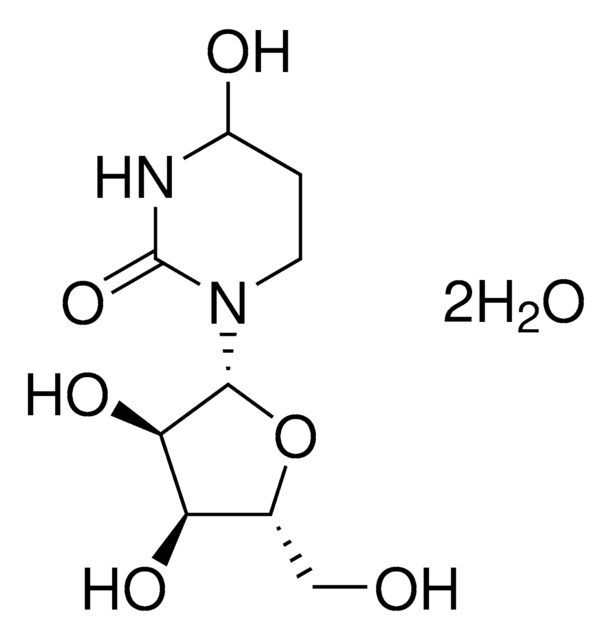
Tetrahydrouridine
Potent competitive inhibitor of cytidine deaminase. Also available as a 100 mM solution in H2O.
Synonym(s):
Tetrahydrouridine
About This Item
Recommended Products
Quality Level
Assay
≥90% (TLC)
form
solid
manufacturer/tradename
Calbiochem®
storage condition
OK to freeze
desiccated (hygroscopic)
protect from light
color
white to off-white
solubility
water: 200 mg/mL
shipped in
wet ice
storage temp.
−20°C
InChI
1S/C9H16N2O6/c12-3-4-6(14)7(15)8(17-4)11-2-1-5(13)10-9(11)16/h4-8,12-15H,1-3H2,(H,10,16)/t4-,5?,6-,7-,8-/m1/s1
InChI key
UCKYOOZPSJFJIZ-XVKVHKPRSA-N
Related Categories
General description
Application
- Oncotherapy resistance explained by Darwinian and Lamarckian models.: This research explores the mechanisms of oncotherapy resistance, combining Darwinian and Lamarckian models to provide a comprehensive understanding. The study highlights the role of Tetrahydrouridine in modulating therapy resistance pathways in cancer treatment (Saunthararajah et al., 2024).
- Neuroendocrine lineage commitment of small cell lung cancers can be leveraged into p53-independent non-cytotoxic therapy.: This study investigates the potential of using Tetrahydrouridine in non-cytotoxic therapies for small cell lung cancers, focusing on neuroendocrine lineage commitment and its implications for treatment efficacy (Biswas et al., 2023).
- In Vitro Interaction of Tetrahydrouridine with Key Human Nucleoside Transporters.: This study explores how Tetrahydrouridine interacts with human nucleoside transporters, shedding light on its mechanisms of action and potential as a therapeutic agent in biochemistry and pharmacology (Säll et al., 2023).
- Mycoplasma infection of cancer cells enhances anti-tumor effect of oxidized methylcytidines.: This research investigates how mycoplasma infection enhances the anti-tumor effects of oxidized methylcytidines, with Tetrahydrouridine playing a crucial role in the observed therapeutic outcomes (Pang et al., 2023).
Packaging
Warning
Reconstitution
Other Notes
Bouffard, D.Y., et al. 1993. Biochem Pharmacol.45, 1857.
Laliberte, J., et al. 1992. Cancer Chemotherap. Pharmacol.30, 7.
Riva, C., et al. 1992. Chemotherapy38, 358.
Yusa, K., et al. 1992. J. Biol. Chem. 267, 16848.
Hanze, A.R. 1967. J. Am. Chem. Soc.89, 6720.
Legal Information
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service