Small Molecule APIs

Small Molecule API Process Development and Manufacturing Services
With decades of expertise, we deliver preclinical to commercial success for your small molecule API program through a consultative and phase appropriate approach. With Millipore® CTDMO Services, tap into our global network to seamlessly execute your custom API projects. From conception to commercialization, we tackle the toughest small molecule API synthesis challenges from grams to commercial quantities.
- Global site network, offering small molecule API process and analytical development, as well as manufacturing resources in North America and Europe
- Batch scalability, supporting early phase quantities to large scale commercial demands
- Integrated and specialized support capabilities, including cryogenic reactions, large scale hydrogenation and continuous flow manufacturing
- Phase appropriate quality and regulatory support throughout the clinical journey
- Sustainability focus, managing your programs environmental footprint through sustainable waste management
- True consultative partnership on small molecule API development and manufacturing programs with strong communication and transparency

As small molecule API programs progress through the clinic, leveraging a well-established commercial partner is critical for success. Our expertise and experience in scaling programs allows us to offer clear consultative recommendations on efficiencies and economies of scale for your program. We provide risk mitigation guidance, advising on hazard analysis and establishing stable supply chains with raw material suppliers.

From pre-clinical to commercial, your small molecule API development and manufacturing program has unique challenges that require an expert partner to bring your molecule to market. Utilizing a phase appropriate approach, we offer exactly what you need when you need it towards your journey to commercialization. With an outstanding regulatory track record, we provide guidance on navigating the complex regulatory landscape.

As small molecule API programs increase in complexity, there is the need for integration with specialized technologies to support a wide variety of programs. Our CDMO site network offers key support such as large-scale hydrogenation and cryogenic reactions. Small molecule API programs can benefit from continuous flow manufacturing for large-volume raw material supply.
INTEGRATED SUPPLY CHAIN FOR API DEVELOPMENT AND PRODUCTION
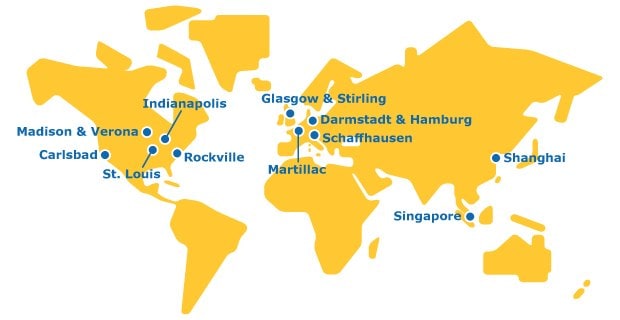
The global footprint of our API contract manufacturing services assures you wide-ranging regulatory and supply expertise, easily accessible through a single trusted partner.
Our API Contract Manufacturing Facility Locations:
GMP contract manufacturing services for large scale API production.
GMP contract manufacturing services for PEGs, lipids, and APIs.
GMP contract manufacturing services for HPAPIs, APIs, advanced intermediates and linker-payloads.
GMP contract manufacturing services for ADC and Bioconjugation, and Small Molecule bio-organics.
Related Video
Large Scale API Production
We take your small molecule API program to the next level with our large scale API production services
Related Webinar
- Large Scale API
In this webinar, explore our latest capacity expansion for large-scale GMP API production located in Darmstadt, Germany. With a commitment to phase-appropriate quality, flexibility, and sustainability, the right CTDMO partner can help navigate the complexities of large-scale programs.
To continue reading please sign in or create an account.
Don't Have An Account?


