Water for BOD & COD Oxygen Demand Testing
Read more about
BOD and COD in water testing
Water use in BOD and COD tests
Impact of water quality on COD analysis
Impact of water quality on BOD analysis
Lab Water Solutions for BOD and COD Oxygen Demand Testing
Related Products
BIOCHEMICAL (OR BIOLOGICAL) OXYGEN DEMAND (BOD) AND CHEMICAL OXYGEN DEMAND (COD) IN WATER TESTING
Oxygen demand (BOD and COD) analyses are commonly used methods to estimate the amount of organic matter in water samples. For example, when treated wastewater is discharged into the environment, it can introduce organic pollutants and deplete dissolved oxygen in the receiving water, leading to negative environmental impact. Depending on the intended use of the water, many regulations and standards include limits on BOD or COD levels. In addition, many industries have established their own internal standards for BOD and COD as part of their environmental management practices.
What is BOD?
BOD evaluates the amount of biodegradable organic material present in wastewater, effluent and polluted waters. The BOD test reflects the amount of dissolved oxygen (DO) consumed by bacteria while oxidizing these materials over a specific amount of time (usually five days). Dissolved oxygen is essential for the life of aquatic fauna and flora, and the BOD test is a measure of the ecological impact that effluent water may have on the receiving body of water (river, lake, etc.). This test is often required in discharge permits, as it is a means of assessing the degree of water pollution.
How is BOD of water measured?
BOD of water is typically measured by placing water samples in sealed containers and incubating in the dark at 20°C for 5 days. During this time, microorganisms consume the organic matter present in the sample and deplete dissolved oxygen. BOD is calculated by subtracting the final dissolved oxygen concentration from the initial value.
What is COD?
COD estimates the theoretical oxygen demand, which is the amount of oxygen required to fully oxidize chemically the organic constituents present in a water sample. The amount of oxygen consumed by a chemical reaction that breaks down organic matter is measured under controlled laboratory conditions. COD is often used to monitor water treatment plant efficiency. It is used as an alternative to BOD when the decomposition of organic matter by microorganisms is limited.
How is COD of water measured?
COD is often measured using a strong oxidant (e.g. potassium dichromate, potassium iodate, potassium permanganate) under acidic conditions. A known excess amount of the oxidant is added to the sample. Once oxidation is complete, the concentration of organics in the sample is calculated by measuring the amount of oxidant remaining in the solution. This is usually done by titration, using an indicator solution. COD is expressed in mg/L, which indicates the mass of oxygen consumed per liter of solution.
Difference between BOD and COD
The COD test only requires 2-3 hours, while the BOD test usually requires 5 days. It measures all organic contaminants, including those that are not biodegradable. There is a relationship between BOD and COD for each specific sample, but it must be established empirically. COD test results can then be used to estimate the BOD of a given sample. Unlike for the BOD test, toxic compounds (such as heavy metals and cyanides) in the samples to be analyzed do not influence the oxidants used in the COD test. Therefore, the COD test can be used to measure the strength of wastes that are too toxic for the BOD test. Some organic molecules (e.g., benzene, pyridine) are relatively resistant to dichromate oxidation and may give a falsely low COD.
Water Use in BOD and COD Tests
Water is used in BOD and COD tests for blank preparation, sample dilutions and glassware rinsing. Purified water is typically used to avoid interference from contaminants that may be present in tap water or other sources of water. It ensures that the water does not contribute to the oxygen demand measurements and that any changes in BOD or COD levels are due to the sample being tested rather than the water itself. The quality of the purified water used can affect the accuracy and precision of the test results.
Impact of Water Quality on COD Analysis
The following water contaminants may have effects on the COD test:
- Ions: Oxidizable inorganic materials may interfere with the determination of COD. They may be oxidized by dichromate and give erroneously high COD results: chlorides are often the most serious source of interference. Nitrites, sulfides and disulfides, sulfites, thiosulfates and ferrous ions may also interfere with the COD test. Manganese can give a positive bias when using photometric detection at 600 nm.
- Organics: Since the COD test measures the oxygen demand of organic compounds in a sample of water, it is important that no outside organic material be accidentally added to the sample to be measured, as it may give falsely high results.
- Bacteria: It is best to minimize bacterial levels in the dilution water, as they may release organics.
Impact of Water Quality on BOD Analysis
The water used to prepare the Dilution Water for the BOD analysis must be of good quality so as not to interfere with the test. Many regulations require that the dissolved oxygen (DO) depletion of dilution water blanks not exceed 0.2 mg/L over the 5-day test period. Various factors may contribute to blank failure; however, it is known that water quality is a major contributor to such failures.
The presence of toxicants or poor seeding material in water samples may lead to falsely low BOD results. Therefore, it is recommended to regularly use a glucose - glutamic acid (GGA) solution as a standard check solution. The oxygen uptake of this solution should be 198 ± 30.5 mg/L. Various water contaminants may influence BOD testing:
- Organics: If organic matter is present in the dilution water, it may increase its oxygen demand, yielding false readings.
- Chlorine or other disinfectants: Chlorine is often present in tap water to control microbial contamination. As chlorine would interfere with the microorganisms used in the BOD test, it should be removed from the water used for the dilutions and the blanks. The same is true of other commonly used tap water disinfectants (chloramine, etc).
- Heavy metals: Water must be free of heavy metals toxic to microorganisms, such as copper, mercury, and cadmium. Like for disinfectants, any compound which may inhibit the growth of microorganisms will have a deleterious effect on the BOD test.
- Bacteria: While bacteria are a necessary component of the BOD test, it is best to minimize their levels in the dissolution water, as they may release organics during storage.
Examples illustrating the impact of water quality on the BOD test1
To better understand the effect of water quality on BOD blanks, three types of water were evaluated:
- Tap water
- Deionized water (DI) obtained using ion-exchange resins
- Ultrapure water produced by an all-in-one Milli-Q® water purification system (similar to a Milli-Q® EQ 7008/16)
For each water type, seven blanks were prepared every week for seven weeks. Disposable BOD bottles were used (made of PET with an inner amorphous carbon coating that prevents oxygen transport), as well as a luminescent dissolved oxygen probe (LBOD).

Figure 1.BOD blank averages and maxima for three water types. Disposable PET bottles and LBOD probe were used to measure dissolved oxygen (DO) depletion. Bars represent the average of 49 blanks, error bars represent standard deviations. Data courtesy of C. Fair, Hach Company (Loveland, CO).
Figure 1 shows BOD blank means and maximums for DO depletion for Tap, DI and Ultrapure water. Average DO depletion for all 3 water types was less than 0.20 mg/L. Standard deviations for Tap and DI were higher than for Ultrapure, while Tap and DI maximum values exceeded the threshold. Tap water, while giving acceptable blank values on average, occasionally gave values above 0.20 mg/L, and the standard deviation was quite high. DI water yielded lower blank values but also yielded failures during the study. Ultrapure water led to even lower blank values and gave the lowest standard deviations. Its important to note that all 49 blanks measured with ultrapure water were well below 0.20 mg/L showing that the Milli-Q® water system produced consistently low blank values and high reproducibility.
The same three types of water (Tap, DI, and Ultrapure water) were evaluated using either the standard glass BOD bottles or disposable PET bottles. Glass BOD bottles should be carefully washed, cleaned with acid and rinsed, while disposable PET bottles do not require washing. For each type of water, the average blank was lower for the PET bottles than for the glass bottles (Figure 2).
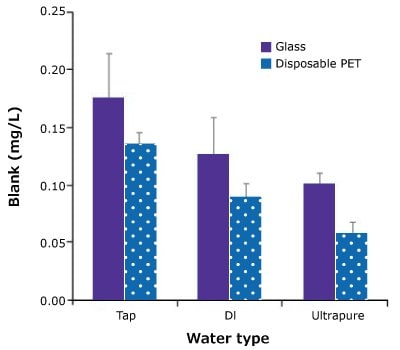
Figure 2. BOD blank averages for glass and PET bottles for Tap, DI, and Ultrapure water types. Bars represent the average of 7 blanks, error bars represent standard deviations. Data courtesy of C. Fair, Hach Company (Loveland, CO).
The ultrapure water produced by the all-in-one water purification system was extremely low in organics and contained no inorganic substances toxic to bacteria, such as chlorine or heavy metals. This study confirmed that the water produced by this system reliably yielded low blank BOD values and good test reproducibility.
Lab Water Solutions for BOD and COD Oxygen Demand Testing
In conclusion, ultrapure (Type 1) water or good quality pure (Type 2) water, purified with a combination of technologies, such as reverse osmosis, ion exchange, and activated carbon is best fitted for BOD or COD tests.
Distilled water may be used to prepare dilution water; however, chlorine may co-distill with water and interfere with the oxygen demand tests. This water would require an additional sodium thiosulfate treatment. Distillation from alkaline permanganate is sometimes recommended, but this purification procedure is quite cumbersome as well. Deionized water, purified with ion exchange resins, may contain organics and microorganisms, thereby causing BOD blank failure. It is therefore not recommended for this test.
A range of solutions adapted to the needs of scientists testing BOD or COD in water is available.
References
To continue reading please sign in or create an account.
Don't Have An Account?