Antibody Drug Conjugate Mimic Enables LC-MS Method Development Without Risk
Kevin Ray, Ben Cutak, Brian Gau
MilliporeSigma, St. Louis, MO
Overview
A non-toxic Cys-conjugated ADC-mimic characterized by top-down, middle-out, and bottom-up LC-MS methods.
Introduction
ADCs conjugated at Cys residues of inter-chain disulfide bonds give mAbs bearing 0-4 pairs of drug-linkers. Characterizing this distribution is paramount to understanding ADC efficacy and process control. Here we show how LC and MS methods may be optimized using a non-toxic surrogate of the ADC, an “ADC-mimic”, that behaves very similarly to the Cys-linked ADC Adcetris (Seattle Genetics). Its dansyl-cadaverine- SMCC mimic-linker imparts a 668 Da mass shift upon conjugation to Cys residues.

Figure 1.
Methods
Native and reduced SEC-MS methods utilized deglycosylation with overnight PNGase F treatment. SEC-MS reduction was 100 mM DTT at 37 °C. RPLC-MS methods (with and without IdeS) used harsher reduction: 7.4 M GndCl and 100 mM DTT at 50 °C. The peptide mapping method utilized a FASP Trypsin/LysC workflow with alkylation by IAA. The M-Class Acquity-Xevo G2 QToF (Waters) was used in all methods except reduced (non-IdeS) RPLC-MS, which used the Acquity-QToF Premier.
Results

Figure 2.ADC-mimic native SEC-MS spectrum. The DAR is the weighted sum of annotated peaks’ intensities. Chromatography: isocratic 100 mM NH4CH3CO2, 70 μL/min, 2 mm x 30 cm TSK Gel SW3000XL.

Figure 3.ADC-mimic and Adcetris reduced SEC-MS spectra. Each DAR is 2x the sum of weighted sums of heavy and light chain conjugate peak intensities. Chromatography: isocratic 30% ACN/0.1% TFA, 70 μL/min, 2 mm x 30 cm TSK Gel SW3000XL.
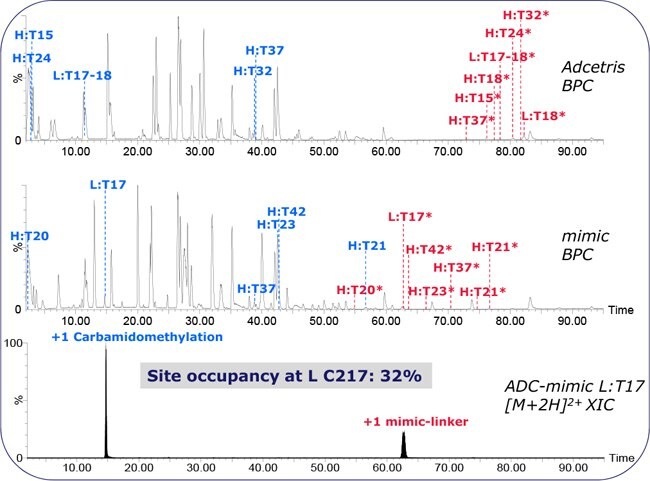
Figure 4.Peptide mapping of Adcetris and the ADC-mimic. Red and blue annotations are of conjugated and unconjugated peptides, respectively. Site occupancy is determined from XIC peak areas. Chromatography: organic 3% - 57% / 2 h, 10 μL/min, 300 μm x 15 cm BIOshell C18 x 2.

Figure 5.RPLC of reduced MAb and ADC-mimic. The MAb Standard (MSQC4) is the MAb component of the ADC-mimic. Chromatography: desalt at 5% organic → 29% - 42% / 13 min, 300 μL/min, 2.1 mm ID x 15 cm BIOshell C4.

Figure 6.RPLC and Fab’ spectra of IdeS/reduced MAb Standard and ADC-mimic. Chromatography: desalt at 5% organic → 31.3% - 32.5% / 4 min → 35% - 38% / 10 min, 40 μL/min, 300 μm x 15 cm BIOshell C4.
Conclusions
We have developed a non-toxic ADC-mimic, with Cys-conjugation to dansyl-cadaverine-SMCC. By native and reduced SEC-MS, its DAR is comparable to Adcetris. The hydrophobicity imparted by the mimic is similar to the highly hydrophobic MMAE-linker of Adcetris. The bottomup, top-down, and middle-out methods here shown for the ADC-mimic are translatable to the characterization of other Cys-conjugated ADCs.
To continue reading please sign in or create an account.
Don't Have An Account?