All Photos(2)
About This Item
Linear Formula:
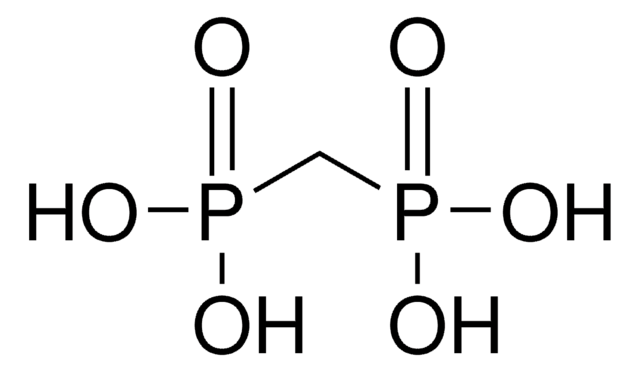
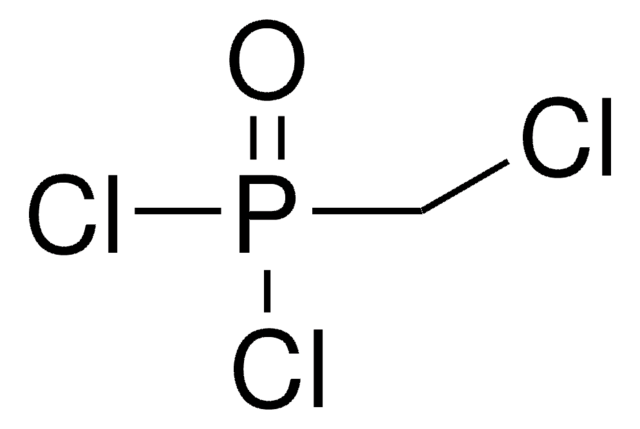
CH2[P(O)Cl2]2
CAS Number:
Molecular Weight:
249.78
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
solid
mp
102-104 °C (lit.)
SMILES string
ClP(Cl)(=O)CP(Cl)(Cl)=O
InChI
1S/CH2Cl4O2P2/c2-8(3,6)1-9(4,5)7/h1H2
InChI key
VRXYCDTWIOCJBH-UHFFFAOYSA-N
Related Categories
General description
Methylenebis(phosphonic dichloride) is an organophosphorus compound that is commonly used in phosphonylation reactions. It is more reactive and the rate of reaction is faster compared to POCl3. This is because the phosphorus center is more electrophilic due to the lack of electron back-donation from the CH2 group.
Application
Methylenebis(phosphonic dichloride) may be used for the following studies:
- Synthesis of mycophenolic methylenebis(phosphonate) derivatives.
- Phosphonylation of nucleosides.
- Preparation of P,P′-partial esters of methylenebisphosphonic acid.
- Synthesis of symmetrical di- and tetra- esters of methylenebisphosphonic acid.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Supplementary Hazards
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Sanjay Bhattarai et al.
Journal of medicinal chemistry, 63(6), 2941-2957 (2020-02-12)
CD73 inhibitors are promising drugs for the (immuno)therapy of cancer. Here, we present the synthesis, structure-activity relationships, and cocrystal structures of novel derivatives of the competitive CD73 inhibitor α,β-methylene-ADP (AOPCP) substituted in the 2-position. Small polar or lipophilic residues increased
Aviran Amir et al.
The Journal of organic chemistry, 78(2), 270-277 (2012-12-05)
A new transformation of methylene-bis(phosphonic dichloride) into tetrathiobisphosphonate derivatives is reported. The reaction of methylene-bis(phosphonic dichloride) with 1,2-ethanedithiol in bromoform in the presence of AlCl(3) formed methylene-bis(1,3,2-dithiaphospholane-2-sulfide), which gave rise to O,O'-diester-methylenediphosphonotetrathioate analogues 1a-k upon reaction with phenols and alkyl
A direct method for the synthesis of nucleoside 5'-methylenebis (phosphonate) s from nucleosides.
Kalek M, et al.
Tetrahedron Letters, 46(!4), 2417-2421 (2005)
Gantla Vidyasagar Reddy et al.
Synthetic communications, 34(2), 331-344 (2004-01-01)
The preparation of partial esters of methylenebisphosphonic acids has been of recent interest due to their potential therapeutic applications. This paper describes a convenient method to prepare symmetrical methylenebis(alkyl hydrogen phosphonates) by the selective cleavage of the corresponding methylenebis(dialkyl phosphonate)
Krzysztof W Pankiewicz et al.
Journal of medicinal chemistry, 45(3), 703-712 (2002-01-25)
Novel mycophenolic adenine dinucleotide (MAD) analogues have been prepared as potential inhibitors of inosine monophosphate dehydrogenase (IMPDH). MAD analogues resemble nicotinamide adenine dinucleotide binding at the cofactor binding domain of IMPDH; however, they cannot participate in hydride transfer and therefore
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service