Assay of Doxepin Hydrochloride acc. to USP Monograph
Lee May May, Application Chemist
Abstract
This study describes a rugged high-performance liquid chromatography (HPLC) method for the assay of Doxepin Hydrochloride, following monograph guidelines as specified by the United States Pharmacopeia (USP). The method is proven to be highly stable with good reproducibility and excellent peak shapes using the fully porous particle (FPP) Purospher® STAR RP-8e column over a wide concentration range of 0.01 to 1.0 mg/mL. The method complies with all USP specifications in terms of resolution, tailing factors, and RSD for both (E)- and (Z)-isomers.
Section Overview:
Introduction
Doxepin hydrochloride is a dibenzoxepin tricyclic compound used as an anti-depressant. The molecular formula of the compound is C19H21NO•HCl and its molecular weight is 315.84 Da. Doxepin HCl is made up of (E) and (Z) geometric isomers. It is a white crystalline powder freely soluble in water, methanol and dichloromethane.1
The assay described below follows that of the USP monograph.2 It uses the fully porous particle (FPP) Purospher® STAR RP-8e column (USP code L7) for an extended doxepin concentration range from 0.01 to 1.0 mg/mL.
Results
System Suitability
USP monograph suitability requirements
- Run time: NLT 2 times the retention time of the (E) isomer
- Resolution: NLT 1.5 between the (E) and (Z) isomers.
- Tailing factor: NMT 2.0 for each (E) and (Z) isomers.
- Relative standard deviation: NMT 2.0% each of the (E)- and (z)-isomers
Acceptance criteria
- (E)-isomer: NLT 81.4% and NMT 88.2%.
- (Z)-isomer: NLT 13.6% and NMT 18.1%.
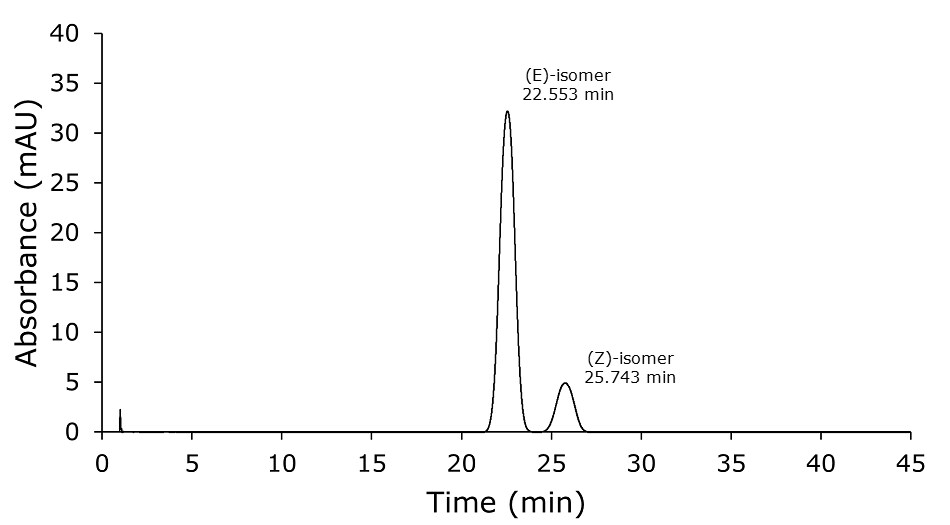
Figure 1.Analysis of 0.1 mg/mL of doxepin HCl in mobile phase. The run time is NLT 2 times the retention time of the (E) isomer (here 22.55 min) following the USP monograph specification.
Linearity & Sensitivity
The linearity of (E)- and (Z)-isomers was demonstrated for 8 concentrations from 0.01 to 1.0 mg/mL with an R2 of 0.9999 (Tables 3 & 4, Figures 2 & 3). This is 10 to 1000% of the doxepin test concentration at 0.1 mg/mL. The compendial guideline for the establishment of an assay linearity is 5 concentrations from 80% to 120% of the test concentration.
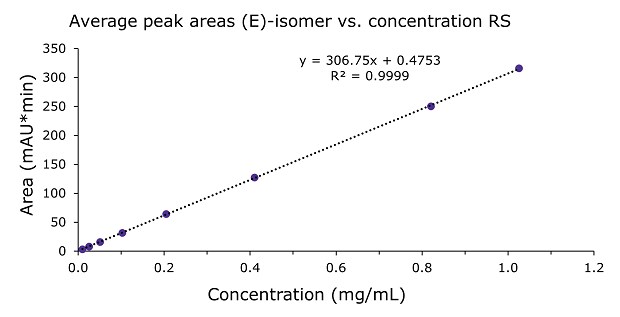
Figure 2.Calibration curve for (E)-isomer from 0.01 to 1.0 mg/mL doxepin HCL RS injections.
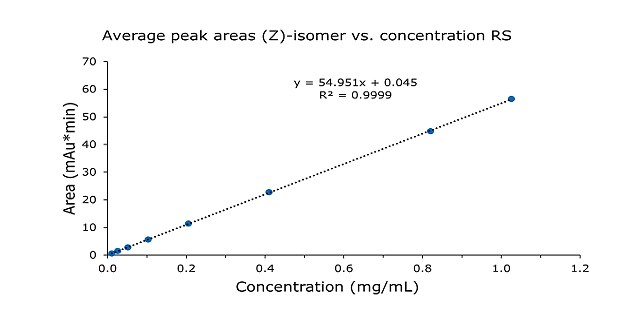
Figure 3.Calibration curve for (Z)-isomer from 0.01 to 1.0 mg/mL doxepin HCL RS injections.
Conclusion
The Purospher® STAR RP-8e column was able to comply with the criteria set out for the assay of doxepin HCl in the USP monograph and demonstrated a solid performance:
- The resolution between (E)- and (Z)-isomers was 1.84. (criteria NLT 1.5)
- The peak symmetry factor of the doxepin (E)-isomer ranged from 0.96 to 1.32 for a concentration from 0.01 to 1 mg/mL. (criteria NMT 2.0)
- % RSD for 5 repeats at 0.1 mg/mL doxepin was 1.47 and 1.44 for the peak areas of the (E)- and (Z)-isomers respectively. (criteria NMT 2%)
- The retention time of (E)- and (Z)-isomers was 22.55 and 25.74 min respectively. (RSD < 2% for n=5)
- LOD and LOQ of the doxepin (E)-isomer was 0.011 and 0.033 mg/mL respectively.*
- LOD and LOQ of the doxepin (Z)-isomer was 0.010 and 0.030 mg/mL respectively.*
*related to the used conc. of doxepin HCl RS
The column was robust and worked well at the low pH of 2.5 at an elevated temperature of 50 °C. Extended repeated injections (data not shown) showed good reproducibility for the retention times so long as the pH is well controlled within ±0.1 units. The column provided excellent peak shapes and was linear for an extended test range – both of which is essential for accurate quantification.
See more applications for Pharmaceutical Analysis and Quality Control
References
Para seguir leyendo, inicie sesión o cree una cuenta.
¿No tiene una cuenta?