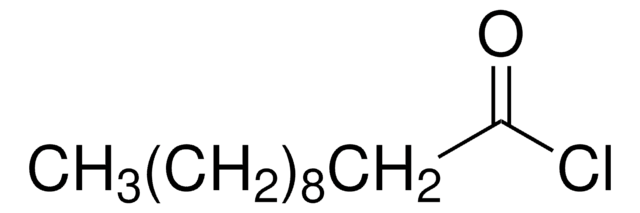468479
4-Pentenoyl chloride
98%
Synonym(s):
4-Pentenoic acid chloride
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
H2C=CHCH2CH2COCl
CAS Number:
Molecular Weight:
118.56
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
bp
125 °C (lit.)
density
1.074 g/mL at 25 °C (lit.)
SMILES string
ClC(=O)CCC=C
InChI
1S/C5H7ClO/c1-2-3-4-5(6)7/h2H,1,3-4H2
InChI key
JDKQTIKEGOOXTJ-UHFFFAOYSA-N
General description
4-Pentenoyl chloride has been identified as a key impurity in 5-chlorovaleroyl chloride (5-CVC). 4-Pentenoyl chloride can be synthesized by reacting thionyl chloride and 4-pentenoic acid.
Application
4-Pentenoyl chloride may be used in the preparation of:
- D-pro-L-derived cyclic peptides
- 4-pentenoylcobalt tricarbonyl
- N-4-pentenoyl-L-cysteine methyl ester
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Liq. 3 - Skin Corr. 1B
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point(F)
80.6 °F - closed cup
Flash Point(C)
27 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
I Nageshwara Rao et al.
The Journal of organic chemistry, 69(6), 2181-2184 (2004-04-03)
An acyclic tripeptide based on a heterochiral D-pro-L-pro template shows a propensity to exist as a 3(10) helical conformation and can be cyclized, via ring-closing metathesis, to the corresponding cyclic tetrapeptides without disrupting the helical conformations in CDCl(3) as well
Synthesis and radical polyaddition of optically active monomers derived from cysteine.
Kudo H, et al.
Macromolecules, 32(25), 8370-8375 (1999)
Alkyl-and Acyl-cobalt Carbonyls Containing Olefinic Unsaturation. Allylcobalt Tricarbonyl and Related Compounds1.
Heck RF and Breslow DS.
Journal of the American Chemical Society, 83(5), 1097-1102 (1961)
Liya Tang et al.
Journal of pharmaceutical and biomedical analysis, 53(3), 309-314 (2010-05-05)
5-Chlorovaleroyl chloride (5-CVC) is commonly used as an alkylating agent in the synthesis of pharmaceutical intermediates, active ingredients, as well as other specialty chemicals. It is critical to monitor the impurities present in 5-CVC as they may have a direct
Norlaily Ahmad et al.
Biomacromolecules, 20(7), 2506-2514 (2019-06-28)
Inflammatory conditions are frequently accompanied by increased levels of active proteases, and there is rising interest in methods for their detection to monitor inflammation in a point of care setting. In this work, new sensor materials for disposable single-step protease
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service