A4375
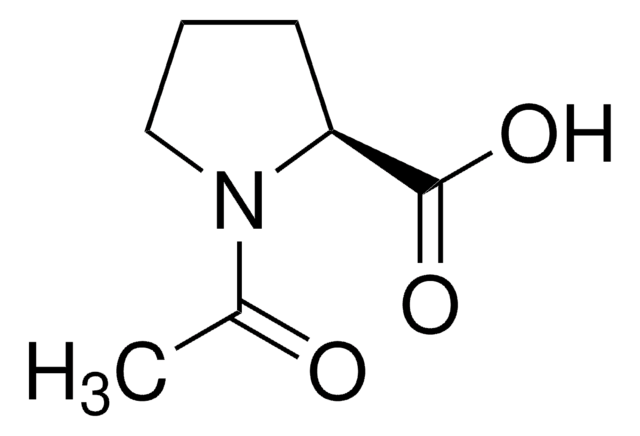
N-Acetyl-D-alanine
≥98% (TLC), suitable for ligand binding assays
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C5H9NO3
CAS Number:
Molecular Weight:
131.13
EC Number:
MDL number:
UNSPSC Code:
12352209
eCl@ss:
32160406
PubChem Substance ID:
NACRES:
NA.26
Recommended Products
product name
N-Acetyl-D-alanine,
Assay
≥98% (TLC)
form
powder
technique(s)
ligand binding assay: suitable
color
white to off-white
storage temp.
−20°C
SMILES string
C[C@@H](NC(C)=O)C(O)=O
InChI
1S/C5H9NO3/c1-3(5(8)9)6-4(2)7/h3H,1-2H3,(H,6,7)(H,8,9)/t3-/m1/s1
InChI key
KTHDTJVBEPMMGL-GSVOUGTGSA-N
Biochem/physiol Actions
N-Acetyl-D-alanine may be used with other D-aminoacylated amino acids as a substrate for the identification, differentiation and characterization of D-aminoacylase(s)/amidohydrolase(s). N-acetyl-D-alanine may be used to study aglycon pocket specific binding on vancomycin.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Yasushi Nitanai et al.
Journal of molecular biology, 385(5), 1422-1432 (2008-11-04)
The crystal structures of three vancomycin complexes with two vancomycin-sensitive cell-wall precursor analogs (diacetyl-Lys-D-Ala-D-Ala and acetyl-D-Ala-D-Ala) and a vancomycin-resistant cell-wall precursor analog (diacetyl-Lys-D-Ala-D-lactate) were determined at atomic resolutions of 1.80 A, 1.07 A, and 0.93 A, respectively. These structures not
P J Loll et al.
Chemistry & biology, 5(5), 293-298 (1998-05-14)
Vancomycin and related glycopeptide antibiotics exert their antimicrobial effect by binding to carboxy-terminal peptide targets in the bacterial cell wall and preventing the biosynthesis of peptidoglycan. Bacteria can resist the action of these agents by replacing the peptide targets with
Ines Slama et al.
Journal of chromatographic science, 40(2), 83-86 (2002-03-08)
The analysis of the binding data of D,L-dansyl amino acids on a vancomycin stationary phase is investigated in relation to the addition of N-acetyl-D-alanine in the mobile phase. This eluent additive acts as a specific competing agent for the aglycone
David J Merkler et al.
Bioorganic & medicinal chemistry, 16(23), 10061-10074 (2008-10-28)
Peptidyl alpha-hydroxylating monooxygenase (PHM) functions in vivo towards the biosynthesis of alpha-amidated peptide hormones in mammals and insects. PHM is a potential target for the development of inhibitors as drugs for the treatment of human disease and as insecticides for
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service