471208
Ethylamine solution
66.0-72.0% in H2O
Synonym(s):
Aminoethane, Monoethylamine
Sign Into View Organizational & Contract Pricing
All Photos(4)
About This Item
Linear Formula:
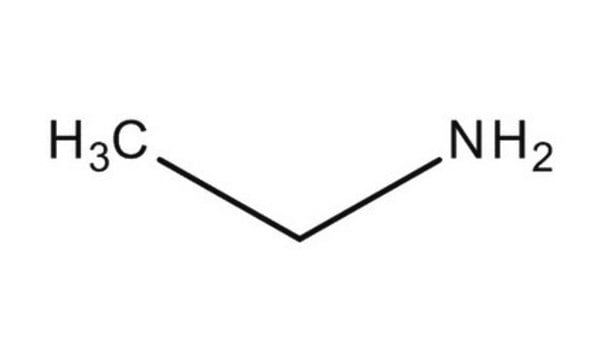
C2H5NH2
CAS Number:
Molecular Weight:
45.08
Beilstein:
505933
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
concentration
66.0-72.0% in H2O
density
0.81 g/mL at 20 °C
functional group
amine
SMILES string
CCN
InChI
1S/C2H7N/c1-2-3/h2-3H2,1H3
InChI key
QUSNBJAOOMFDIB-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Ethylamine solution is used in the preparation of N-ethyl triazineepiperazine copolymer (ETPC), as a hydrophobic flame retardant material. It is also used in the modification of poly(tetramethylene oxide)/silica hybrid composites.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 4 Oral - Eye Dam. 1 - Flam. Liq. 2 - Skin Corr. 1 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point(F)
71.6 °F
Flash Point(C)
22 °C
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Synthesis of N-ethyl triazine-piperazine copolymer and flame retardancy and water resistance of intumescent flame retardant polypropylene.
Yang, Kun et al.
Polymer Degradation and Stability, 98(7), 1397-1406 (2013)
Modification of an inorganic-organic hybrid composite: effect of ethylamine.
Brennan, Anthony B and Miller, Thomas M
Chemistry of Materials, 6(3), 262-267 (1994)
Câline Christine et al.
Organic & biomolecular chemistry, 10(46), 9183-9190 (2012-10-23)
The synthesis of a phosphonated acyclic bifunctional chelate L* for the labeling of biomaterial is described. L* is based on a pyridine backbone, functionalized in ortho positions by aminomethyl-bis-methylphosphonic acids, and, in the para position, by a side chain containing
Song Yang et al.
Journal of chromatography. A, 1216(15), 3280-3289 (2009-03-10)
Complementary methods using liquid chromatography-tandem quadrupole mass spectrometry (LC-MS/MS) and comprehensive two-dimensional gas chromatography-time-of-flight mass spectrometry (GCxGC-TOF-MS) were developed and applied to determine targeted metabolites involved in central carbon metabolism [including tricarboxylic acid cycle, serine cycle, ethylmalonyl-coenzyme A (ethylmalonyl-CoA) pathway
Lisa Vasicek et al.
Analytical chemistry, 81(19), 7876-7884 (2009-09-03)
Electron transfer dissociation (ETD) has proven to be a promising new ion activation method for proteomics applications due to its ability to generate c- and z-type fragment ions in comparison to the y- and b-type ions produced upon the more
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service