推荐产品
等级
analytical standard
质量水平
保质期
limited shelf life, expiry date on the label
技术
HPLC: suitable
gas chromatography (GC): suitable
沸点
319 °C/773 mmHg (lit.)
mp
114-117 °C (lit.)
应用
environmental
包装形式
neat
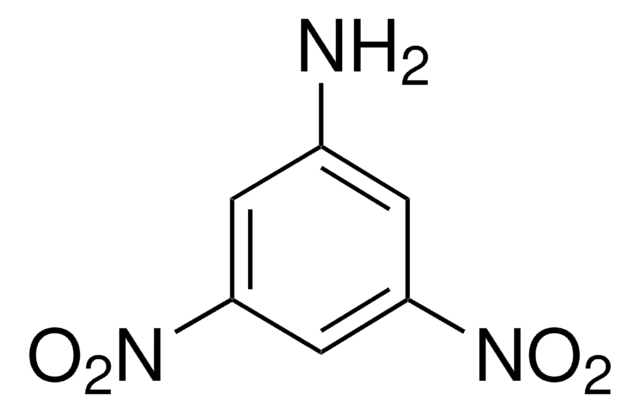
SMILES字符串
[O-][N+](=O)c1ccccc1[N+]([O-])=O
InChI
1S/C6H4N2O4/c9-7(10)5-3-1-2-4-6(5)8(11)12/h1-4H
InChI key
IZUKQUVSCNEFMJ-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般描述
1,2-Dinitrobenzene belongs to the class of nitroaromatic compounds, which are utilized for the manufacture of pesticides, explosives, polymers, etc. They can pose a potential risk to the environment because of their toxic nature.
应用
1,2-Dinitrobenzene has been used as an analytical standard for the determination of the analyte in organic explosives investigated during the forensic analysis by liquid chromatography-atmospheric pressure chemical ionization mass spectrometry (LC-APCI-MS). It may be used as an analytical standard for the determination of the analyte in aqueous samples, environmental samples and human biological samples by various chromatographic techniques.
Refer to the product′s Certificate of Analysis for more information on a suitable instrument technique. Contact Technical Service for further support.
推荐产品
Find a digital Reference Material for this product available on our online platform ChemisTwin® for NMR. You can use this digital equivalent on ChemisTwin® for your sample identity confirmation and compound quantification (with digital external standard). An NMR spectrum of this substance can be viewed and an online comparison against your sample can be performed with a few mouseclicks. Learn more here and start your free trial.
警示用语:
Danger
危险分类
Acute Tox. 1 Dermal - Acute Tox. 2 Inhalation - Acute Tox. 2 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - STOT RE 2
储存分类代码
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
闪点(°F)
302.0 °F - closed cup
闪点(°C)
150 °C - closed cup
个人防护装备
dust mask type N95 (US), Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
M E Walsh
Talanta, 54(3), 427-438 (2008-10-31)
Hazardous waste site characterization, forensic investigations, and land mine detection are scenarios where soils may be collected and analyzed for traces of nitroaromatic, nitramine, and nitrate ester explosives. These thermally labile analytes are traditionally determined by high-performance liquid chromatography (HPLC);
Application of DLLME based on the solidification of floating organic droplets for the determination of dinitrobenzenes in aqueous samples
Wu Y, et al.
Chromatographia, 72(7-8), 695-699 (2010)
K Asaoka et al.
Journal of biochemistry, 94(5), 1685-1688 (1983-11-01)
Conditions have been examined for the use of o-dinitrobenzene as a substrate for colorimetric assay of glutathione S-transferases. Activities can be determined by measuring nitrite released enzymatically from the substrate using a diazo-coupling method with N-(1-naphthyl)ethylenediamine dihydrochloride and sulfanilamide. The
A L Wu et al.
The Journal of biological chemistry, 271(6), 2914-2920 (1996-02-09)
Glutathione-dependent detoxification reactions are catalyzed by the enzyme glutathione S-transferase and are important in drug resistance in organisms ranging from bacteria to humans. The yeast Issatchenkia orientalis expresses a glutathione S-transferase (GST) protein that is induced when the GST substrate
T Hirayama et al.
Mutation research, 191(2), 73-78 (1987-06-01)
3,4-Dinitrobiphenyl derivatives were mutagenic in Salmonella typhimurium TA98, TA98/1,8-DNP6 and in TA98NR. We describe here the specific reactivity of 3,4-dinitrobiphenyl derivatives with diluted sodium hydroxide solution and the determination of the amounts of released nitrous ion. 3,4-Dinitrobiphenyl derivatives begin to
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门