所有图片(3)
About This Item
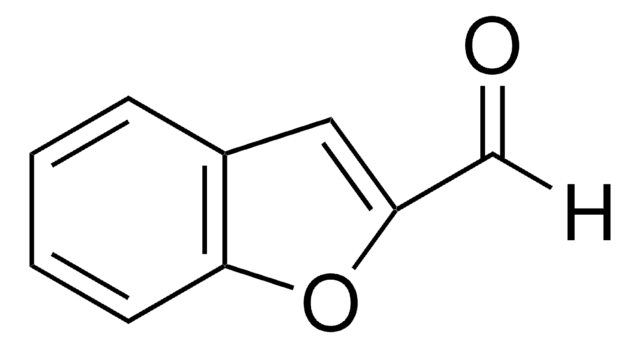
经验公式(希尔记法):
C9H6OS
CAS号:
分子量:
162.21
MDL號碼:
分類程式碼代碼:
12352100
PubChem物質ID:
NACRES:
NA.22
推荐产品
化驗
95%
bp
166 °C/20 mmHg (lit.)
mp
53-57 °C (lit.)
SMILES 字串
O=Cc1csc2ccccc12
InChI
1S/C9H6OS/c10-5-7-6-11-9-4-2-1-3-8(7)9/h1-6H
InChI 密鑰
WDJLPQCBTBZTRH-UHFFFAOYSA-N
一般說明
Thianaphthene-3-carboxaldehyde, also known as benzo[b]thiophene-3-carboxaldehyde, can be synthesized from 3-methyl-benzo[b]thiophene. It undergoes phosphine-free palladium coupling with aryl halides to form 2-arylbenzo[b]thiophenes.
應用
Thianaphthene-3-carboxaldehyde (Benzo[b]thiophene-3-carboxaldehyde) may be used as a starting material in the multi-step synthesis of anthra[2,3-b:7,6-b′]bis[1benzothiophenes (ABBTs).
It may be used in the synthesis of :
It may be used in the synthesis of :
- 6-(N,N-dimethylamino)-2-(benzo[b]thiophen-3-yl)quinazolin-4-one
- 6-(pyrrolidin-1-yl)-2-(benzo[b]thiophen-3-yl)quinazolin-4-one
- (Z)-2-(benzo[b]thiophen-3-ylmethylene)-1-azabicyclo[2.2.2]octan-3-one
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
其他客户在看
M J Hour et al.
British journal of pharmacology, 169(7), 1574-1586 (2013-05-04)
Our previous study demonstrated that 6-(pyrrolidin-1-yl)-2-(3-methoxyphenyl)quinazolin-4-one (HMJ38) was a potent anti-tubulin agent. Here, HMJ38 was used as a lead compound to develop more potent anti-cancer agents and to examine the anti-cancer mechanisms. Using computer-aided drug design, 2-aryl-6-substituted quinazolinones (MJ compounds)
(Z)-2-(Benzo[b] thiophen-3-ylmethylene)-1-azabicyclo [2.2.2] octan-3-one.
Sonar VN, et al.
Acta Crystallographica Section E, Structure Reports Online, 59(11), o1726-o1728 (2003)
An efficient phosphine-free palladium coupling for the synthesis of new 2-arylbenzo[b]thiophenes.
Chabert JFD, et al.
Tetrahedron, 60(14), 3221-3230 (2004)
Synthesis and properties of isomerically pure anthrabisbenzothiophenes.
Lehnherr D, et al.
Organic Letters, 14(1), 62-65 (2011)
Benzo[b]thiophene derivatives. VIII. Benzo[b]thiophene-3-earboxaldehyde and derivatives.
Campaigne E and Neiss ES.
Journal of Heterocyclic Chemistry, 3(1), 46-50 (1966)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门![苯并[b]噻吩-2-甲醛 97%](/deepweb/assets/sigmaaldrich/product/structures/321/060/32405a4e-5720-4c6d-91cf-115c747270c4/640/32405a4e-5720-4c6d-91cf-115c747270c4.png)