Released N-Glycan Analysis of a Therapeutic Antibody Using BIOshell™ Glycan Column
Maricar Dube, Analytical Sciences Liaison, Cory Muraco, HPLC Product Manager, Judy Boland, Senior R&D Scientist, Amber Henry, R&D Scientist
Merck
Introduction
Therapeutic monoclonal antibodies (mAbs) have seen explosive growth since the first mAb was approved by the US FDA over thirty years ago. In fact, over the past five years, therapeutic antibodies have become the best-selling drugs,1 and they continue to grow in terms of new approvals and targets.2
Monoclonal antibodies are target specific, which means that they have high efficacy few side effects. However, compared to chemically synthesized small molecule therapies, mAbs are considerably more complex owing to their size and nature of their development and production. These mAbs are expressed using recombinant technologies in mammalian cell lines or other expression systems, giving rise to heterogeneity mainly through post-translational modifications (PTMs).3 These PTMs need to be characterized as they affect the efficacy, stability, half-life, and safety of mAbs.
Glycosylation is one of the most common and important PTMs for mAbs. Glycosylation of antibody involves the attachment of glycans at specific sites on a protein, most commonly at asparagine (Asn) (N-linked glycosylation) or serine/threonine (Ser/Thr) (O-linked glycosylation) amino acid residues.4 There are four levels of analytical approaches to N-glycan analysis: intact glycoproteins, glycopeptides, released glycans, and monosaccharide analyses.5 This article focuses on the analysis of released N-glycans by high-pressure liquid chromatography (HPLC).
The steps for a released N-glycan profiling are outlined in the workflow below. The N-linked glycans are released by an amidase such as peptide-N-glycosidase F (PNGase F). The released glycans are then labeled with a fluorescent tag, like aminobenzamide (2-AB) or procainamide (4-amino-N-[2-(diethylamino)ethyl]benzamide). Prior to the HPLC analysis, a clean-up step is needed to remove excess tags and salts. Hydrophilic interaction liquid chromatography (HILIC) is a proven technique for separating and quantifying over other HPLC methods (e.g. reverse phase, anion exchange).4
Workflow for released N-glycan analysis by HPLC
Glycan Release
Labelling
Cleanup
HPLC Analysis
This article uses a BIOshell™ Glycan HPLC column to analyze cetuximab (Erbitux®) N-glycans labeled with procainamide. BIOshell™ Glycan HPLC columns are specifically engineered to deliver a fast, high-resolution, and reproducible glycan identification using HILIC.
Experimental Conditions
Glycan Release and Labeling
PNGase Fast Kit was used for glycan release with FASP (filter-aided sample prep). The released glycans were labeled using procainamide with reductive amination.
Sample Cleanup
The labeled samples were diluted with 99% acetonitrile and loaded into the conditioned Discovery® Glycan SPE cartridges. They were allowed to flow through the cartridges slowly, using gravity (slight pressure or vacuum would also be suitable), making sure that the sample was entirely in the resin bed. The cartridges were then washed five times with 99% acetonitrile using a vacuum. After the washing step, 20% acetonitrile was added to each cartridge and allowed to elute slowly, using gravity (applying slight pressure or vacuum would also be suitable). After all the eluent had passed into the resin bed, vacuum was used to evacuate all liquid from the SPE into the collection tube. The eluted labeled glycans were then dried by vacuum centrifugation. Table 1 shows the SPE conditions.
HPLC Analysis
The dried and labeled glycans were solubilized by dissolving in 50 µL of 75% ACN / 25% 75 mM ammonium formate pH 4.4, vortexing for 2 min, followed by centrifugation at 16,000 x g for 2 min. Table 2 shows the chromatographic conditions.
Results
In this study, cetuximab was used as a model therapeutic mAb to analyze released N-glycans. This mAb is a chimeric mouse-human IgG1 monoclonal antibody, against the epidermal growth factor receptor (EGFR). Cetuximab is used to treat head, neck, as well as colorectal cancers. The antibody is N-glycosylated both in the fragment crystallizable (Fc) and fragment antigen binding (Fab) regions. There are numerous studies and reports showing the attachment of N-glycans to mAbs and the subsequent effect of the attachment on various biological and physicochemical processes, leading to safety and quality issues.6,7 Some of the processes affected by glycosylation include enhancement of the structural integrity of the mAb, serum half-life, antibody-dependent cellular toxicity (ADCC), anti-inflammatory activities, immunity, and antigen recognition. This clearly indicates the important need for understanding glycosylation patterns.
BIOshell™ HPLC columns are based on Fused-Core® particles (also called core-shell or superficially porous particles (SPPs)), characterized by a thin, porous shell of high-purity silica surrounding a solid, silica core. This design allows for a shorter diffusion path compared to traditional fully porous particles, as illustrated in Figure 1. The short diffusion path accelerates the mass transfer of solutes (“C” term in the van Deemter equation), concomitantly resulting in high column efficiency.

Figure 1.Fused-Core® particles have shorter diffusion paths compared to traditional Fully Porous Particles (FFP).
The stationary phase in the BIOshell™ Glycan column is a highly polar ligand that has five hydroxyl (-OH) groups tethered to the silica via a novel and proprietary chemical linkage. This unique column chemistry is suitable for the analysis of oligosaccharides, particularly for protein-linked glycans using the typical mobile phases for HILIC of oligosaccharides.
A fluorescence chromatogram of procainamide-labeled cetuximab glycans is shown in Figure 2. The BIOshell™ Glycan column was able to elucidate the complex glycosylation of this mAb. The corresponding glycans were identified by MS analysis (mass spectrometry data not shown).

Figure 2.Fluorescence chromatogram of procainamide-labeled cetuximab glycans on BIOshell™ Glycan column. LC-MS was used to characterize each peak (chromatogram not shown). A rapid release glycan protocol was used.
Successful analysis of N-linked glycans by HPLC requires an efficient and reproducible glycan release step. The traditional protocol involves multiple wash steps and overnight digestion of the native or denatured mAb. The protocol described in the experimental section of this article is a fast protocol that uses a proprietary detergent-based buffer for rapid deglycosylation of N-linked glycans using PNGase F. In this fast protocol, complete release of N-glycans is achieved in a 15-minute incubation, compared to the traditional overnight digestion.
In another experiment, the BIOshell™ Glycan column was used to compare released glycans from cetuximab using three glycan release protocols:
- Traditional overnight protocol, denatured using guanidine hydrochloride
- Fast protocol, non-reduced (rapid deglycosylation)
- Fast protocol, reduced (rapid deglycosylation under reducing conditions using 2-mercaptoethanol)
The results are shown in Figure 3.
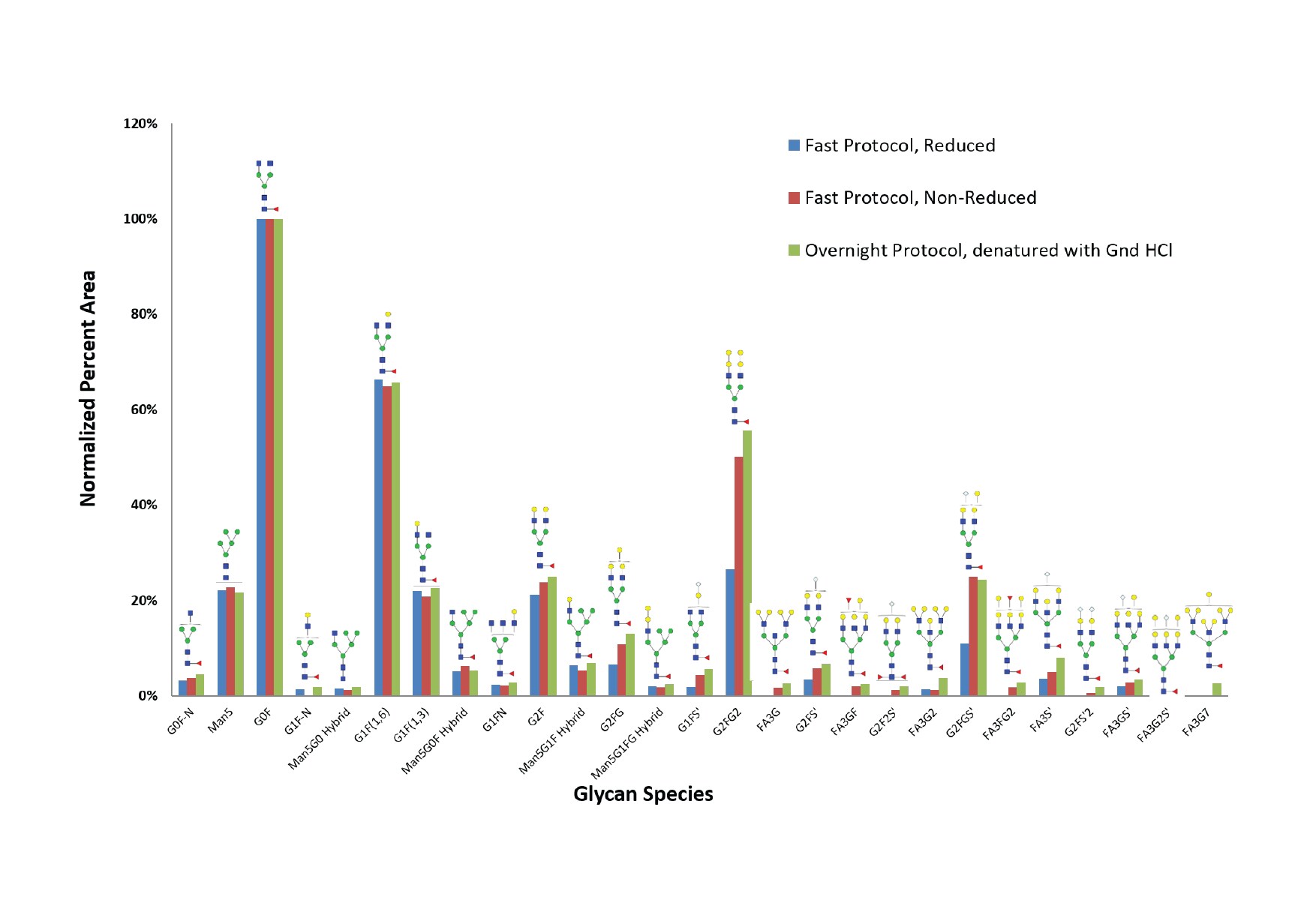
Figure 3.Comparison of cetuximab glycan distribution using three glycan release protocols: Fast (reduced), Fast (non-reduced), and Traditional overnight protocol. A BIOshell™ Glycan column was used in all three samples.
With this particular analyte (cetuximab), all three protocols were found to be equally efficient for some glycan species, such as G0F-N, Man5, G0F, G1(F1,6), Man5G0F Hybrid, and G1F (1,3). But there were some glycans that were not efficiently released with the fast protocol when a reducing step using 2-mercaptoethanol was included, for example, G1F5’, G2FG2, FA3G, FA3GF, G2F2S’, and G2FGS’. Indeed, the use of 2-mercaptoethanol in the denaturing step is not required for most proteins while using the fast protocol. But there are proteins, like RNAse B, that seem to require it. As part of optimizing a method for released glycan analysis, the fast protocol must be tested with and without 2-mercaptoethanol to check for the best results.
It is also worth noting that some proteins are not amenable to fast deglycosylation techniques. When working with mAbs without established protocols for glycan analysis, it is best to compare the results of the traditional overnight digest to the fast/rapid digestion protocol.8
Conclusion
Characterizing and monitoring the glycosylation pattern of a therapeutic mAb is required by regulatory authorities to ensure the efficacy and safety of the drug. While analysis and identification of glycans can be challenging because of their structural complexity, this article has shown that a BIOshell™ Glycan HPLC column was able to elucidate the complex glycosylation of cetuximab after an appropriate glycan release and labeling protocol. Another key consideration in glycan analysis is the deglycosylation protocol. While there is a fast method that significantly saves time, it is recommended to compare the results with the traditional overnight digestion and choose the one that gives more efficient deglycosylation.
References
To continue reading please sign in or create an account.
Don't Have An Account?