Tetrakis(dimethylamino)ethylene (TDAE)
Mark Redlich
In 2001, Professor William Dolbier, Jr., at the University of Florida reported1 an approach to nucleophilic trifluoromethylation based on the generation of a trifluoromethyl anion using CF3I in the presence of a powerful twoelectron reductant, tetrakis(dimethylamino)ethylene (TDAE) (Scheme 1).

Scheme 1
Over the ensuing years, Dolbier’s group has developed numerous reactions featuring this reagent system, including the trifluoromethylation of aldehydes and ketones,1 cyclic sulfates,2 disulfides and diselenides,3 and imines1 (Scheme 2). In many cases, the TDAE/CF3I method provided results that complement other nucleophilic trifluoromethylation reagents. Recently, Dolbier has expanded the use of his system to perfluoroalkylation,4 and was able to achieve similar results as with CF3I; however, when the perfluorobutyl system was used, yields tended to decrease noticeably.
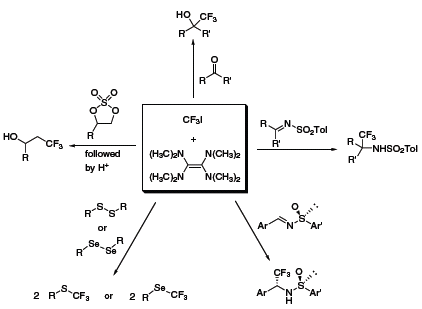
Scheme 2
TDAE has a reducing power comparable to zinc metal and as such has also been used as a reducing agent in organic synthesis. Recently, Vanelle and coworkers have exploited this reductive property to obtain epoxides from aldehydes and 2-(dibromomethyl)quinoxaline (Scheme 3),5 as well as α-chloroketones from aldehydes and 2-(trichloromethyl)-substituted azaheterocycles (Scheme 4).6 The Nishiyama group at Kansai University in Osaka has also used TDAE to obtain 1,2,3,4-tetrahydronaphthalenes from 1,2-bis(bromomethyl)arenes and olefins,7 and 1,4-diketones or diesters through reductive coupling of α-bromoketones or esters (Scheme 5).8
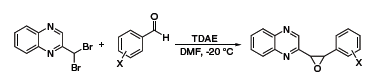
Scheme 3
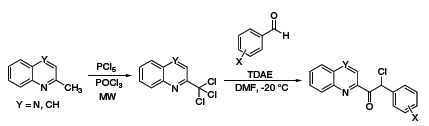
Scheme 4
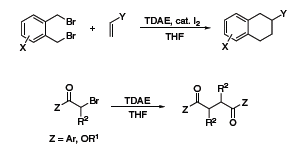
Scheme 5
References
To continue reading please sign in or create an account.
Don't Have An Account?