Polymerizing via ATRP
The following two procedures describe two ATRP polymerization reactions as performed by Prof. Dave Hadddleton′s research group at the University of Warwick.
Read more about

Figure 1.Polymerization of methylmethacrylate (MMA) with N-propyl-2-pyridylmethanimine, where 1a = N-propyl-2-pyridylmethanimine
The polymerization is carried out under oxygen free conditions. First the transition metal salt is deoxygenated and then the solution, followed by adding initiator to start the polymerization:
- Cu(I)Br (0.134 g, 9.32 × 10-4 moles, Product No. 212865) and a dry magnetic stir bar were added to a dry Schlenk flask (or round-bottom flask).
- The tube is sealed with a rubber septum and deoxygenated with three freeze, pump, thaw cycles using N2.
- The polymerization solution was prepared by adding the following reagents/solvents to the flask under N2:
- Toluene (10 mL)
- MMA (10 mL, 9.36 × 10-2 moles, Product No. M55909)
- N-propyl-2-pyridylmethanimine (0.279 g, 1.87 × 10-3 moles)
- The Schlenk tube is subjected to three additional freeze, pump, thaw cycles and then subsequently heated to 90 °C with constant stirring.
- Once the reaction temperature is reached the initiator ethyl- 2-bromoisobutyrate (0.136 mL, 9.36 × 10-4 moles, Product No. E14403) is added under N2 (t = 0) and the polymerization reaction begins.
Samples can be removed at 30-60 minute intervals using a degassed syringe and analyzed by GC or NMR to determine the extent of conversion and SEC for molecular weight and polydispersity (PDI). After 300 minutes, the poly(methylmethacrylate) (PMMA) is isolated with a final conversion of 89.3 %. Mn = 8,760 g mol-1 (theoretical Mn = 8,930 g mol-1), PDI = 1.20.
Styrene Polymerization with N-pentyl 2-pyridyl methanimine
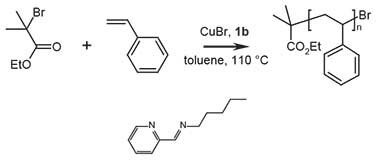
Figure 2.Polymerization of styrene with N-pentyl-2-pyridylmethanimine, where 1b = N-pentyl-2-pyridylmethanimine
The polymerization is carried out under oxygen free conditions. First the transition metal salt is deoxygenated, and then the monomer and initiator are added to the solution and deoxygenated, followed by adding ligand to start the polymerization:
- Cu(I)Br (0.131 g, 9.1 × 10-4 moles) and a dry magnetic stir bar were added to a dry Schlenk flask (or round-bottom flask).
- The tube is sealed with a rubber septum and deoxygenated with three freeze, pump, thaw cycles using N2.
- The polymerization solution was prepared as follows by adding the following to the flask under N2:
- Xylene (20 mL)
- Styrene (10 mL, 8.73 × 10-2 moles, Product No. 240869)
- Ethyl-2-bromoisobutyrate (0.13 mL, 9.1 × 10-4 mol)
- The flask is subjected to an additional three freeze, pump, thaw cycles and subsequently heated to 110 oC with constant stirring.
- Once the reaction temperature is reached, N-pentyl-2-pyridylmethanimine (0.28 mL, 1.8 × 10-3 mol) is added under N2 (t = 0).
Samples can be removed at 30-60 minute intervals using a degassed syringe and analysed by GC or NMR for conversion and SEC for molecular weight and polydispersity (PDI). The reaction was stopped after 360 minutes, with a final conversion of 77.9 %. Mn = 5,270 (theoretical Mn = 7,900 g mol-1), PDI = 1.29.
To continue reading please sign in or create an account.
Don't Have An Account?