The Du Bois group at Stanford University has made substantial progress within the field of Rh-catalyzed C–H amination via oxidative cyclization of carbamate, sulfamate, sulfamide, urea, and guanidine substrates to give 1,2- and 1,3-heteroatom motifs masked in the form of 5- and 6-membered ring heterocycles (Scheme 1).1-8 The cyclization can occur with a high degree of stereospecificity for optically active substrates.

Scheme 1.
Additionally, heterocyclic urea and guanidines themselves appear as structural elements in pharmacologically interesting substrates such as NK1 receptor antagonists, toxins, and bromopyrrole metabolites (Figure 1).3,8

Figure 1.
A variety of Rh-carboxylate catalysts exhibit at least some effectiveness in the urea and guanidine cyclizations, but the most promising results are obtained using the combination of catalytic Rh2(esp)2 with substrates bearing 2,2,2-trichloroethoxysulfonyl (Tces) protection at nitrogen. Installation of the urea or guanidine residue, followed by cyclization can be accomplished through the use of several reagents as outlined in Schemes 2–6.3 Mitsunobu reaction of an alcohol with Tces-protected urea gives the cyclization precursor in good yield (Scheme 2). Subsequent treatment of this species with Rh2(esp)2 in the presence of PhI(OAc)2 and MgO affords the cyclized product in good to excellent yields for substrates containing tertiary or benzylic b-C–H centers (Scheme 3).

Scheme 2.

Scheme 3.
Tces-protected guanidines are easily prepared by of the reaction of an amine with one of two reagents derived from S,S-dimethyl-N- (2,2,2-trichloroethoxysulfonyl)carbonimidodithionate (Scheme 4).
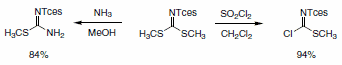
Scheme 4.
The isothiourea reacts with most primary amines in water at 100 °C to give the Tces-protected guanidine derivative (Scheme 5). Alternatively, more functionalized amines can be reacted with the carbonchloroimidodithioate reagent to give an intermediate pseudothiourea that can be transformed into the desired guanidine using HMDS as an ammonia source.

Scheme 5.
Treatment of these Tces-protected guanidines under the standard oxidative cyclization reaction conditions furnishes the cyclized products in good yield (Scheme 6). Like the urea cyclizations, the reaction is most successful for substrates containing tertiary or benzylic β-C–H centers. Removal of the protecting group can be effected in high-yield using Zn metal and methanolic acetic acid.

Scheme 6.
References
Para seguir leyendo, inicie sesión o cree una cuenta.
¿No tiene una cuenta?