LC/MS Analysis of Clenbuterol In Plasma on Astec® CHIROBIOTIC® T with Phospholipid Removal using HybridSPE® Phospholipid
Materiales
Analytical column
Application
LC/MS Analysis of Clenbuterol Enantiomers in Plasma on Astec® CHIROBIOTIC® T after SPE using HybridSPE®-Phospholipid
application for HPLCSPE tube or plate
standard
CONDITIONS
sample preparation
SPE (Solid Phase Extraction)
sample/matrix
rat plasma spiked with clenbuterol enantiomers at 10 ng/mL
SPE well plate
HybridSPE-Precipitation 96-well Plate, 50 mg/well (575656-U)
sample addition
100 μL spiked rat plasma followed by 300 μL 1% formic acid in acetonitrile. Mix by vortexing the HybridSPE-PPT plate briefly.
elution
apply vacuum
column
CHIROBIOTIC T, 10 cm x 2.1 mm I.D., 5 μm particles (12018AST)
mobile phase
10 mM ammonium formate in methanol
flow rate
0.3 mL/min
column temp.
30 °C
detector
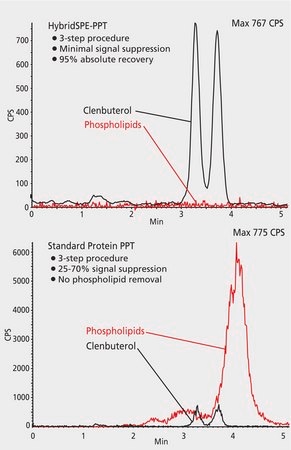
ABI 3200 QT; ESI(+), MRM: 184/104 m/z (phospholipids); 277.2/203.1 m/z (clenbuterol)
injection
10 μL
Descripción
Application
The HybridSPE method provides significant improvement in LC-MS baseline.
Legal Information
Astec is a registered trademark of Merck KGaA, Darmstadt, Germany
CHIROBIOTIC is a registered trademark of Sigma-Aldrich Co. LLC
HybridSPE is a registered trademark of Merck KGaA, Darmstadt, Germany