E6376
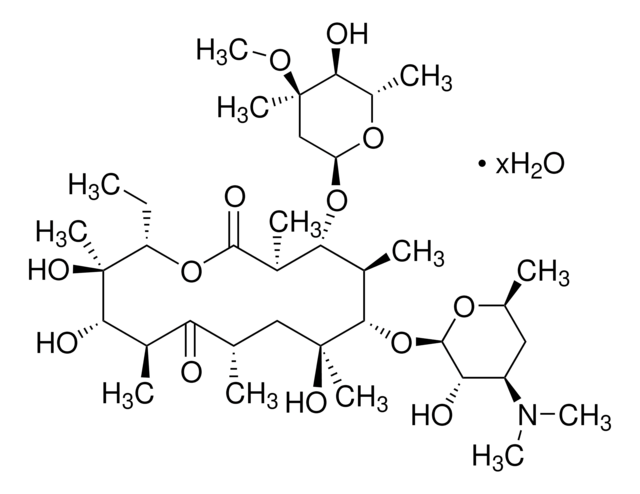
Erythromycin
potency: ≥850 μg per mg
Sinónimos:
Erythromycin A
About This Item
Productos recomendados
biological source
Streptomyces erythreus
Quality Level
form
powder
potency
≥850 μg per mg
color
white
solubility
ethanol: 50 mg/mL, clear to slightly hazy, colorless to faintly yellow
antibiotic activity spectrum
Gram-negative bacteria
Gram-positive bacteria
mode of action
protein synthesis | interferes
SMILES string
CC[C@H]1OC(=O)[C@H](C)[C@@H](O[C@H]2C[C@@](C)(OC)[C@@H](O)[C@H](C)O2)[C@H](C)[C@@H](O[C@@H]3O[C@H](C)C[C@@H]([C@H]3O)N(C)C)[C@](C)(O)C[C@@H](C)C(=O)[C@H](C)[C@@H](O)[C@]1(C)O
InChI
1S/C37H67NO13/c1-14-25-37(10,45)30(41)20(4)27(39)18(2)16-35(8,44)32(51-34-28(40)24(38(11)12)15-19(3)47-34)21(5)29(22(6)33(43)49-25)50-26-17-36(9,46-13)31(42)23(7)48-26/h18-26,28-32,34,40-42,44-45H,14-17H2,1-13H3/t18-,19-,20+,21+,22-,23+,24+,25-,26+,28-,29+,30-,31+,32-,34+,35-,36-,37-/m1/s1
InChI key
ULGZDMOVFRHVEP-RWJQBGPGSA-N
Gene Information
human ... ABCB1(5243) , CYP3A4(1576) , MLNR(2862)
mouse ... Abcb1a(18671) , Abcb1b(18669)
¿Está buscando productos similares? Visita Guía de comparación de productos
General description
Application
Erythromycin is an antibiotic produced by growth of certain strains of Streptomyces erythreus. This product is composed largely of erythromycin A with small amounts of erythromycins B and C and is recommended for concentration at 100 mg/L. Concentrations between 50 and 200 mg/L have also proven effective in controlling bacterial growth. Erythromycin has been used as a motilin receptor agonist, to block respiratory glycoconjugate secretion in human airways in vitro, and for selecting plasmid-cured and recombinant lactococcus lactis MG1363 strains.
Biochem/physiol Actions
Antimicrobial Spectrum: This product acts against both gram-negative and gram-positive bacteria.
Caution
Preparation Note
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
Antibiotics targeting bacterial ribosomes disrupt protein synthesis, a key process in bacterial growth inhibition.
Antibiotics targeting bacterial ribosomes disrupt protein synthesis, a key process in bacterial growth inhibition.
Antibiotics targeting bacterial ribosomes disrupt protein synthesis, a key process in bacterial growth inhibition.
Antibiotics targeting bacterial ribosomes disrupt protein synthesis, a key process in bacterial growth inhibition.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico