All Photos(2)
About This Item
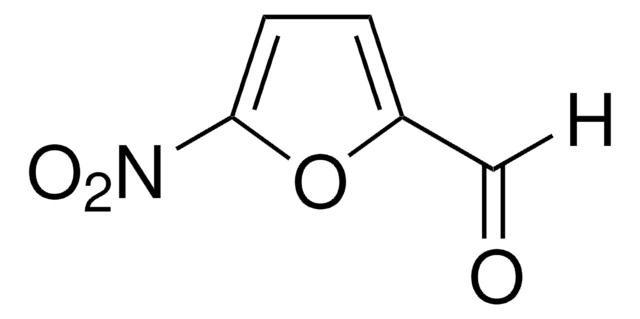
Empirical Formula (Hill Notation):
C5H3NO3S
CAS Number:
Molecular Weight:
157.15
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
solid
mp
75-77 °C (lit.)
solubility
acetone: soluble 1%, clear, yellow
SMILES string
[H]C(=O)c1ccc(s1)[N+]([O-])=O
InChI
1S/C5H3NO3S/c7-3-4-1-2-5(10-4)6(8)9/h1-3H
InChI key
CHTSWZNXEKOLPM-UHFFFAOYSA-N
General description
Diastereoselectivity in [4+2] cycloaddition of 1-methoxy-2-methyl-3-(trimethylsiloxy)-1,3-pentadiene with 5-nitro-2-thiophenecarboxaldehyde was investigated.
Application
5-Nitro-2-thiophenecarboxaldehyde was used in preparation of 2, 3-dihydro-2-(5-nitro-2-thienyl) quinazolin-4-(1H)-ones and various novel oxime ether derivatives, anti-protozoan agents.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Ali Almasirad et al.
Iranian journal of pharmaceutical research : IJPR, 10(4), 727-731 (2011-10-01)
A series of new 2-(phenylthio) benzoylarylhydrazones were synthesized by acid-catalyzed condensation of hydrazide 3 with corresponding aldehydes. The chemical structures of the compounds were elucidated by FT-IR, (1)H-NMR and Mass spectra. All newly synthesized compounds were evaluated for their antimycobacterial
Antibacterial 2,3-dihydro-2-(5-nitro-2-thienyl)-quinazolin-4(1H)-ones.
R J Alaimo et al.
Journal of medicinal chemistry, 15(3), 335-336 (1972-03-01)
Michael P Doyle et al.
Proceedings of the National Academy of Sciences of the United States of America, 101(15), 5391-5395 (2004-04-03)
Chiral dirhodium(II) carboxamidates are highly efficient catalysts for reactions between a variety of aldehydes and activated dienes. Catalyst loadings as low at 0.01 mol % have been realized with enantioselectivities up to 97%. Kinetic investigations reveal a pronounced electronic influence
Synthesis and< i> in vitro</i> anti-protozoan activity of new 5-nitrothiophene oxime ether derivatives.
Delmas F, et al.
European Journal of Medicinal Chemistry, 28(1), 23-27 (1993)
Jian Xu et al.
Food chemistry, 221, 1530-1538 (2016-12-17)
We synthesized a series of 4- or 5-functionalized TCT derivatives (1-12) and investigated their inhibitory activities and mechanisms on tyrosinase by using Spectrofluorimetry, 1H and 13C NMR titration and IR spectra. The results of the fluorescence spectra and NMR titrations
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service