Recommended Products
sterility
non-sterile
Quality Level
form
lyophilized powder
extent of labeling
~30 mol steroid per mol BSA
solubility
phosphate buffer: 0.90-1.10 mg/mL, faintly hazy to hazy, colorless to faintly yellow
shipped in
ambient
storage temp.
2-8°C
General description
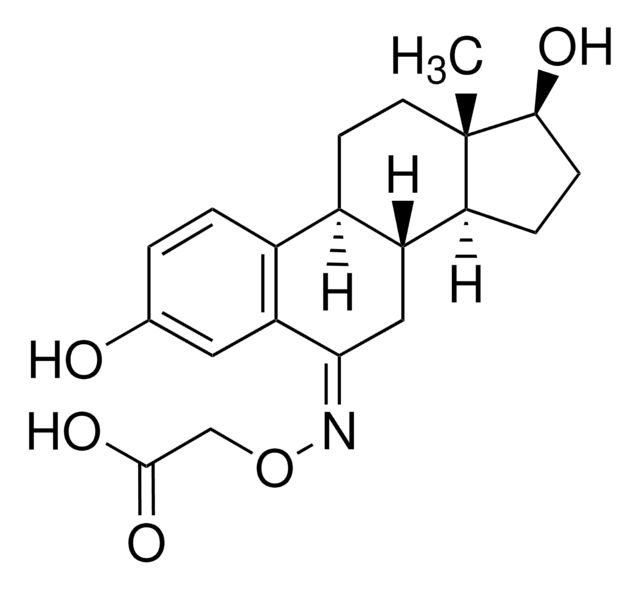
β-Estradiol 6-(O-carboxymethyl)oxime: BSA is an estradiol conjugate and is membrane-impermeable. BSA is linked to the estradiol at position 6 to form the β-estradiol 6-(O-carboxymethyl) oxime: BSA conjugate.
Application
β-Estradiol 6-(O-carboxymethyl)oxime: BSA has been used:
- to test its effect on the N-methyl-D-aspartate receptor (NMDAR) currents in female dorsal root ganglion (DRG) neurons
- to coat microplates for the detection of plasma 17β-Estradiol levels using indirect enzyme-linked immunosorbent assay (ELISA)
- to test its effect on MutL homolog 1 in colorectal cancer lines
Biochem/physiol Actions
β-Estradiol 6-(O-carboxymethyl)oxime: BSA mimics estrogen and favors amyloid precursor protein α (sAPPα) secretion in vitro studies with primary rat cortical neurons. It also stimulates mitogen-activated protein kinase (MAPK) activity in human umbilical vein endothelial cells
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Carc. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Adrián Tintos et al.
Ecotoxicology and environmental safety, 66(2), 139-147 (2006-02-10)
To assess the effects of naphthalene on liver intermediary metabolism and plasma steroid hormones, immature female rainbow trout (Oncorhynchus mykiss), in a first experiment, were intraperitoneally injected (2 microLg(-1)) with vegetable oil alone (control) or containing naphthalene (10 and 50
Sex-dependent differences in the activity and modulation of N-methyl-d-aspartic acid receptors in rat dorsal root ganglia neurons.
McRoberts, et al.
Neuroscience, 148, 1015-1020 (2018)
Sun Zhang et al.
Acta pharmacologica Sinica, 26(2), 171-176 (2005-01-25)
To investigate the mechanism of the action of estrogen, which stimulates the release of secreted amyloid precursor protein alpha (sAPP(alpha)) and decreases the generation of amyloid-beta protein (A(beta)), a dominant component in senile plaques in the brains of Alzheimer's disease
Claudio R F Marinho et al.
PloS one, 4(5), e5630-e5630 (2009-05-23)
Pregnancy-associated malaria (PAM) is associated with placenta pathology and poor pregnancy outcome but the mechanisms that control the malaria parasite expansion in pregnancy are still poorly understood and not amenable for study in human subjects. Here, we used a set
K S Russell et al.
Proceedings of the National Academy of Sciences of the United States of America, 97(11), 5930-5935 (2000-05-24)
Estrogen induces both rapid and delayed effects on the cardiovascular system. The early effects take place within minutes (e.g., changes in vasomotor tone) and are mediated through rapid intracellular signaling pathways; whereas the delayed effects (e.g., remodeling or lipid alterations)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service