All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C8H8O
CAS Number:
Molecular Weight:
120.15
Beilstein:
111928
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
liquid
refractive index
n20/D 1.549 (lit.)
bp
188-189 °C (lit.)
solubility
alcohol: soluble
carbon disulfide: soluble
chloroform: soluble
diethyl ether: soluble
density
1.065 g/mL at 25 °C (lit.)
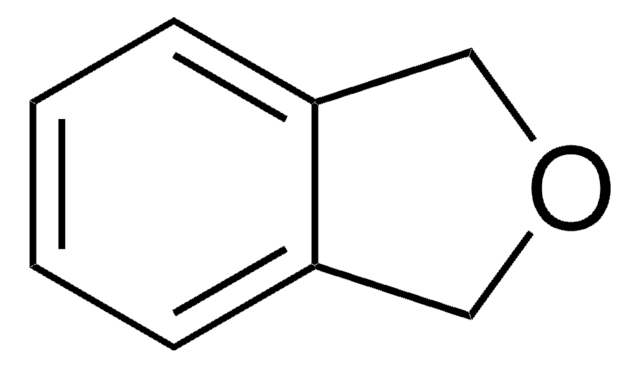
SMILES string
C1Cc2ccccc2O1
InChI
1S/C8H8O/c1-2-4-8-7(3-1)5-6-9-8/h1-4H,5-6H2
InChI key
HBEDSQVIWPRPAY-UHFFFAOYSA-N
Gene Information
human ... CYP1A2(1544)
Looking for similar products? Visit Product Comparison Guide
General description
Biotransformation of 2,3-dihydrobenzofuran using intact cells of Pseudomonas putida UV4 has been investigated. 2,3-Dihydrobenzofuran is the intermediate formed during catalytic hydrodeoxygenation of benzofuran.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
152.6 °F - closed cup
Flash Point(C)
67 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Guo-Hua Chu et al.
Bioorganic & medicinal chemistry letters, 15(23), 5114-5119 (2005-10-06)
Two novel chemical classes of kappa opioid receptor agonists, chroman-2-carboxamide derivatives and 2,3-dihydrobenzofuran-2-carboxamide derivatives, were synthesized. These agents exhibited high and selective affinity for the kappa opioid receptor.
Catalytic hydrodeoxygenation of benzofuran and o-ethylphenol.
Lee C-L and Ollis DF.
J. Catal., 87(2), 325-331 (1984)
Xiangtai Meng et al.
Organic letters, 11(1), 137-140 (2008-12-05)
A new bifunctional phosphine catalyst, (2'-hydroxy-biphenyl-2-yl)-diethylphosphane (LBBA-1), was developed for the highly stereoselective synthesis of cis-2,3-dihydrobenzofurans via an aza-Morita-Baylis-Hillman/umpolung addition domino reaction of salicyl N-thiophosphinyl imines with electron-deficient allenes. Dual activation of both nucleophile and electrophile by the bifunctional catalyst
Structures and stereochemical assignments of some novel chiral synthons derived from the biotransformation of 2, 3-dihydrobenzofuran and benzofuran by Pseudomonas putida.
Boyd DR, et al.
Tetrahedron Asymmetry, 4(6), 1307-1324 (1993)
Leticia Jiménez-González et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 12(34), 8762-8769 (2006-09-06)
2,3-Dihydrobenzofurans can be diastereoselectively prepared by condensation of aromatic aldehydes with 2,3-dihydrobenzoxasilepines under the catalysis of Ag(I) complexes, and in the presence of a source of fluoride ion. The application of this strategy by using chiral catalysts leads to a
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service