A0664
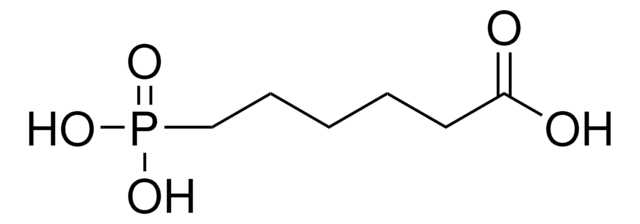
4-Aminobutylphosphonic acid
≥99%
Synonym(s):
P-(4-aminobutyl)-phosphonic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C4H12NO3P
CAS Number:
Molecular Weight:
153.12
MDL number:
UNSPSC Code:
12352106
PubChem Substance ID:
NACRES:
NA.26
Recommended Products
Quality Level
Assay
≥99%
form
powder
color
white
application(s)
detection
SMILES string
NCCCCP(O)(O)=O
InChI
1S/C4H12NO3P/c5-3-1-2-4-9(6,7)8/h1-5H2,(H2,6,7,8)
InChI key
IDPXFPYGZRMMKZ-UHFFFAOYSA-N
Biochem/physiol Actions
4-Aminobutylphosphonic acid, a GABA B receptor ligand, is used in studies on the regulation of prolactin (PRL) secretion and differential GABA receptor research.
4-aminobutylphosphonic acid, the phosphonic acid analogue of delta-aminovaleric acid, inhibits gamma-aminobutyric acid B (GABA-B receptor) binding without influencing either isoproterenol- or forskolin-stimulated cyclic AMP production.
Linkage
Analog of δ-aminovaleric acid
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
H P De Koning et al.
Endocrinology, 132(2), 674-681 (1993-02-01)
The activity of many endocrine cells is regulated by gamma-aminobutyric acid (GABA). The effects of GABA are mediated by GABAA and/or GABAB receptors. While GABAB receptors in the central nervous system have now been extensively characterized, little is known of
N G Ternan et al.
FEMS microbiology letters, 184(2), 237-240 (2000-03-14)
A strain of the yeast Kluyveromyces fragilis was screened for its ability to utilize a range of synthetic and natural organophosphonate compounds as the sole source of phosphorus, nitrogen or carbon. Only 4-aminobutylphosphonate was utilized as sole nitrogen source with
R M Woodward et al.
Molecular pharmacology, 43(4), 609-625 (1993-04-01)
Poly(A)+ RNA from mammalian retina expresses bicuculline/baclofen-insensitive gamma-aminobutyric acid (GABA) receptors in Xenopus oocytes with properties similar to those of homooligomeric GABA rho 1 receptors. The pharmacological profile of these rho-like receptors was extended by measuring sensitivities to various GABAA
J Ong et al.
Naunyn-Schmiedeberg's archives of pharmacology, 357(4), 408-412 (1998-05-30)
The effects of five phosphonic derivatives of GABA on the release of [3H]-GABA from rat neocortical slices, preloaded with [3H]-GABA, were investigated. Phaclofen and 4-aminobutylphosphonic acid (4-ABPA) increased the overflow of [3H] evoked by electrical stimulation (2 Hz) in a
L S Wong et al.
Pharmacology, biochemistry, and behavior, 38(4), 829-835 (1991-04-01)
The application of 1.2 and 12.0 micrograms/side of the GABAA receptor agonist 3-aminopropane sulphonic acid bilaterally into the nucleus accumbens (Acb) of rats nonsignificantly depressed locomotor activity as assessed in automated Animex activity cages, while the highest dose (60 micrograms/side)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service