HPLC Analysis of CYP3A Inhibitors on Ascentis® Express RP-Amide
Materials
analytical column
Product No.
Description
Pricing
mobile phase component
Product No.
Description
Pricing
standard
Product No.
Description
Pricing
CONDITIONS
column
Ascentis Express RP-Amide, 10 cm x 2.1 mm I.D., 2.7 μm particles (53913-U)
mobile phase
[A] 20 mM ammonium formate in 50:50 (v/v) water:acetonitrile
flow rate
0.6 mL/min
column temp.
35 °C
detector
ESI(+), m/z 100-600
injection
1 μL
Description
Analysis Note
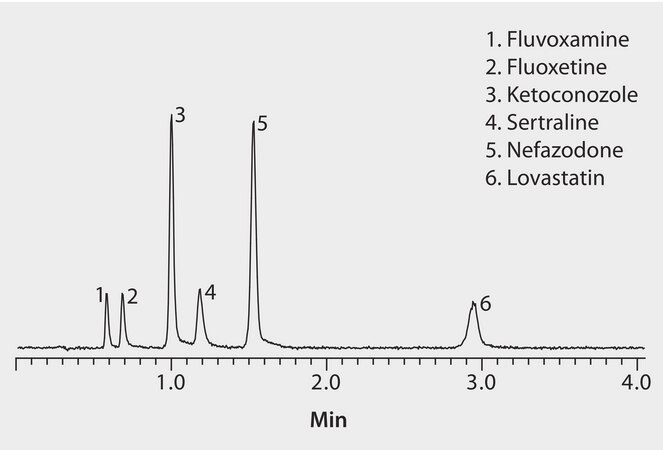
This application demonstrates the suitability of the Ascentis Express RP Amide for the analysis of CYP3A Inhibitors.
Legal Information
Ascentis is a registered trademark of Merck KGaA, Darmstadt, Germany